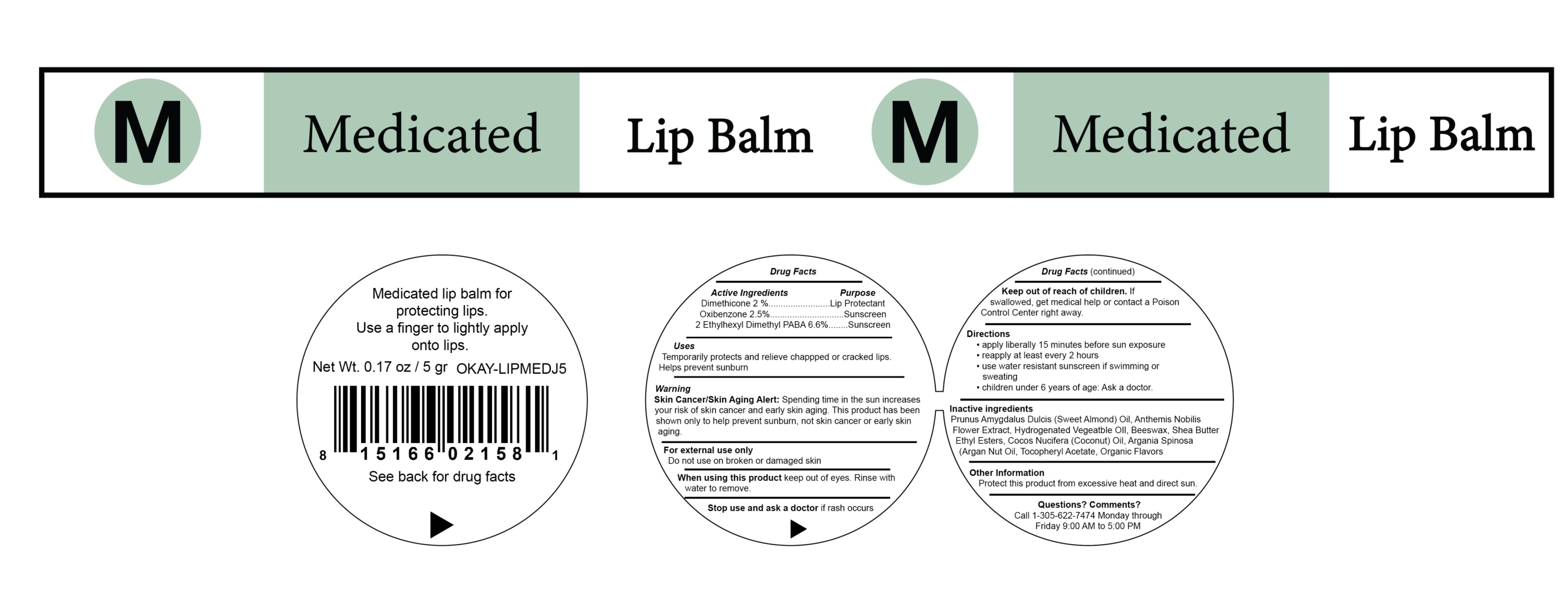
MEDICATED LIP BALM by Xtreme Tools International, Inc MEDICATED LIP BALM
MEDICATED LIP BALM by
Drug Labeling and Warnings
MEDICATED LIP BALM by is a Otc medication manufactured, distributed, or labeled by Xtreme Tools International, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MEDICATED LIP BALM- dimethicone, oxybenzone, and 2-ethylhexyl dimethyl paba cream
Xtreme Tools International, Inc
----------
MEDICATED LIP BALM
Purpose
Dimethicone 2.0% - Lip protectant
Oxybenzone 2.5%- Sunscreen
2-Ethylhexyl Dimethyl PABA 6.6%- Sunscreen
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 years of age: Ask a doctor
| MEDICATED LIP BALM
dimethicone, oxybenzone, and 2-ethylhexyl dimethyl paba cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Xtreme Tools International, Inc (125398904) |
| Registrant - Xtreme Tools International, Inc (125398904) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Xtreme Tools International, Inc | 125398904 | manufacture(74553-004) | |
Revised: 3/2025
Document Id: 340ce4a9-9388-47c9-ac3e-236b320ba326
Set id: b139e34b-4fe8-41d9-e053-2a95a90aaa5a
Version: 4
Effective Time: 20250312