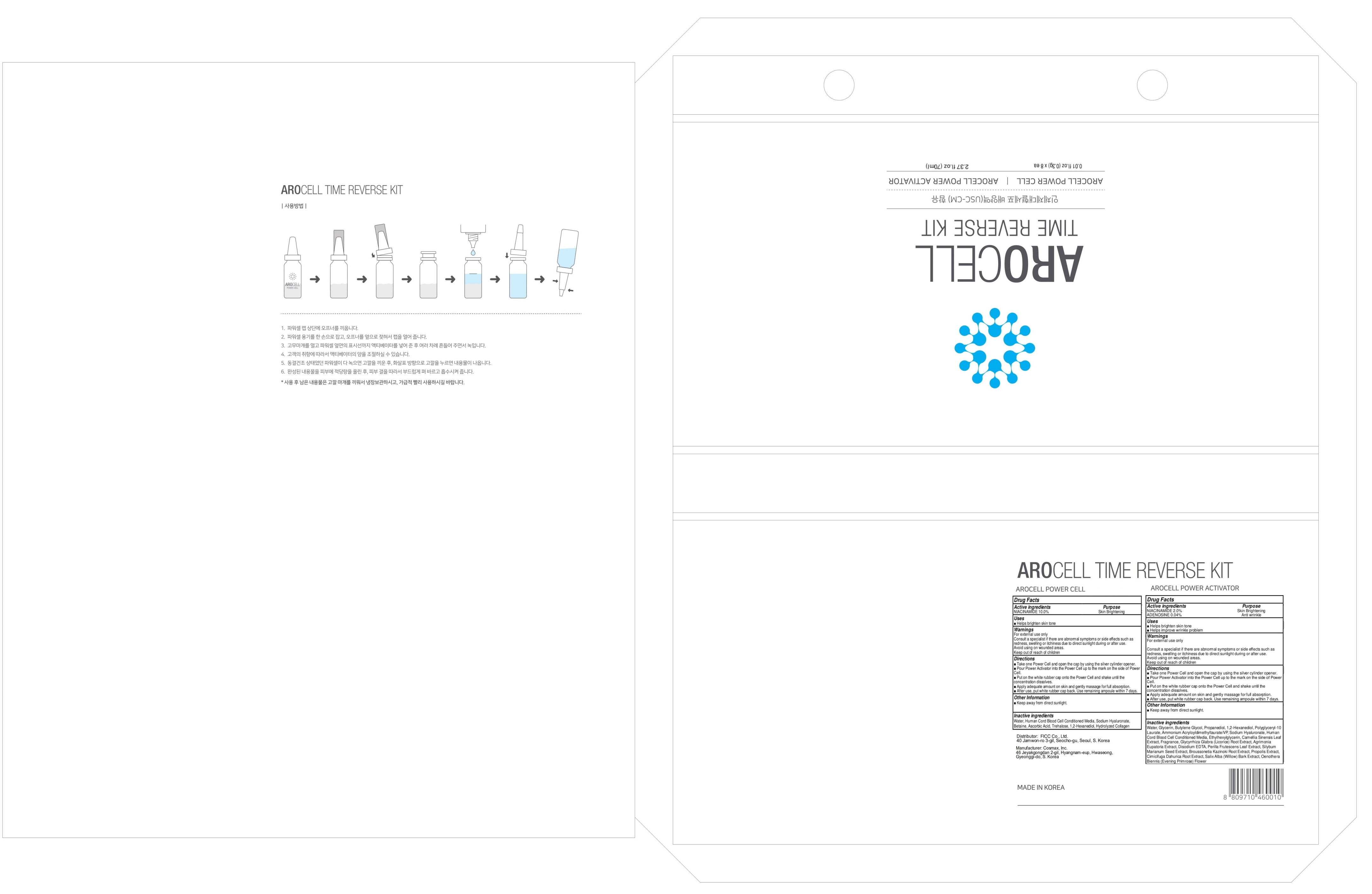
ACTIVE INGREDIENTS
[AROCELL POWER ACTIVATOR]
NIACINAMIDE 2.0%
ADENOSINE 0.04%
[AROCELL POWER CELL]
NIACINAMIDE 10.0%
INACTIVE INGREDIENTS
[AROCELL POWER ACTIVATOR]
Water, Glycerin, Butylene Glycol, Propanediol, 1,2-Hexanediol, Polyglyceryl-10 Laurate, Ammonium Acryloyldimethyltaurate/VP, Sodium Hyaluronate, Human Cord Blood Cell Conditioned Media, Ethylhexylglycerin, Camellia Sinensis Leaf Extract, Fragrance, Glycyrrhiza Glabra (Licorice) Root Extract, Agrimonia Eupatoria Extract, Disodium EDTA, Perilla Frutescens Leaf Extract, Silybum Marianum Seed Extract, Broussonetia Kazinoki Root Extract, Propolis Extract, Cimicifuga Dahurica Root Extract, Salix Alba (Willow) Bark Extract, Oenothera Biennis (Evening Primrose) Flower
[AROCELL POWER CELL]
Water, Human Cord Blood Cell Conditioned Media, Sodium Hyaluronate, Betaine, Ascorbic Acid, Trehalose, 1,2-Hexanediol, Hydrolyzed Collagen
PURPOSE
[AROCELL POWER ACTIVATOR]
Skin Brightening
Anti wrinkle
[AROCELL POWER CELL]
Skin Brightening
WARNINGS
For external use only
Consult a specialist if there are abnormal symptoms or side effects such as redness, swelling or itchiness due to direct sunlight during or after use.
Avoid using on wounded areas.
Keep out of reach of children
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
Uses
[AROCELL POWER ACTIVATOR]
■ Helps brighten skin tone
■ Helps improve wrinkle problem
[AROCELL POWER CELL]
■ Helps brighten skin tone
Directions
■ Take one Power Cell and open the cap by using the silver cylinder opener.
■ Pour Power Activator into the Power Cell up to the mark on the side of Power Cell.
■ Put on the white rubber cap onto the Power Cell and shake until the concentration dissolves.
■ Apply adequate amount on skin and gently massage for full absorption.
■ After use, put white rubber cap back. Use remaining ampoule within 7 days.
Other Information
■ Keep away from direct sunlight.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL