Hydraboost Oil-Free Sunscreen SPF 40
Hydraboost Oil-Free Sunscreen SPF 40 by
Drug Labeling and Warnings
Hydraboost Oil-Free Sunscreen SPF 40 by is a Otc medication manufactured, distributed, or labeled by Bluemercury, Biogenesis, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYDRABOOST OIL-FREE SUNSCREEN SPF 40- homosalate, octisalate, and avobenzone lotion
Bluemercury
----------
Hydraboost Oil-Free Sunscreen SPF 40
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10am-2pm.
- Wear long-sleeve shirts, pants, hats & sunglasses.
- Children under 6 months of age: Ask a doctor.
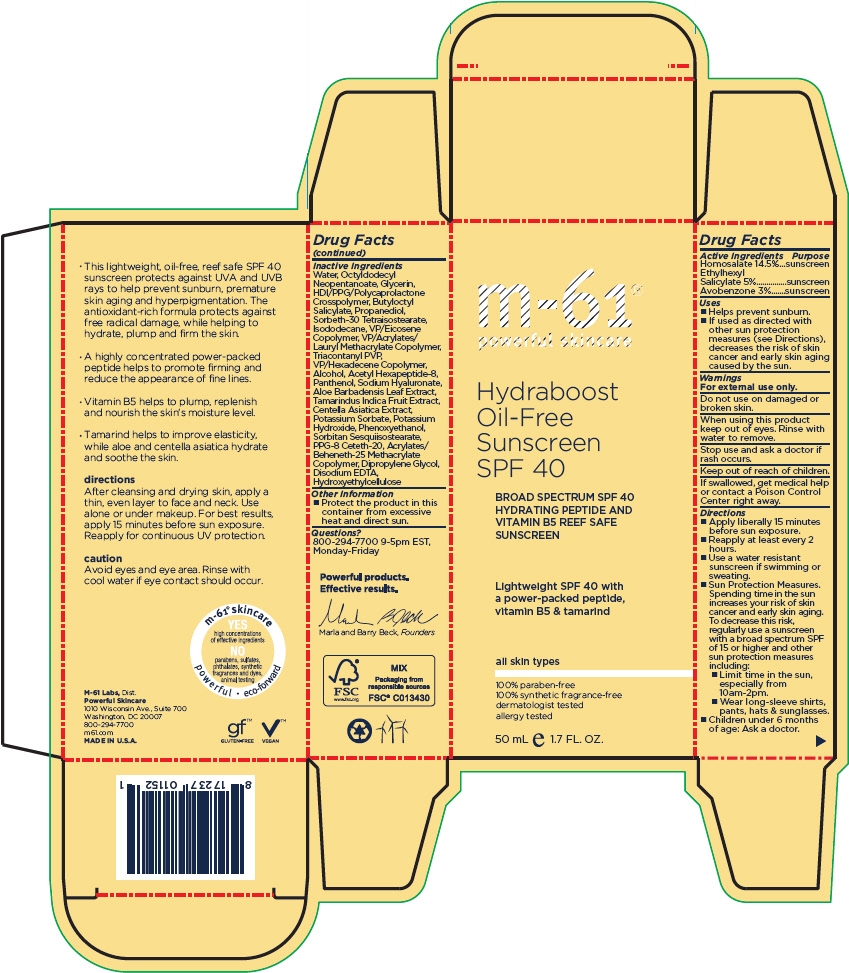
Inactive Ingredients
Water, Octyldodecyl Neopentanoate, Glycerin, HDI/PPG/Polycaprolactone Crosspolymer, Butyloctyl Salicylate, Propanediol, Sorbeth-30 Tetraisostearate, Isododecane, VP/Eicosene Copolymer, VP/Acrylates/Lauryl Methacrylate Copolymer, Triacontanyl PVP, VP/Hexadecene Copolymer, Alcohol, Acetyl Hexapeptide-8, Panthenol, Sodium Hyaluronate, Aloe Barbadensis Leaf Extract, Tamarindus Indica Fruit Extract, Centella Asiatica Extract, Potassium Sorbate, Potassium Hydroxide, Phenoxyethanol, Sorbitan Sesquiisostearate, PPG-8 Ceteth-20, Acrylates/Beheneth-25 Methacrylate Copolymer, Dipropylene Glycol, Disodium EDTA, Hydroxyethylcellulose
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
m-61
®
powerful skincare
Hydraboost
Oil-Free
Sunscreen
SPF 40
BROAD SPECTRUM SPF 40
HYDRATING PEPTIDE AND
VITAMIN B5 REEF SAFE
SUNSCREEN
Lightweight SPF 40 with
a power-packed peptide,
vitamin B5 & tamarind
all skin types
100% paraben-free
100% synthetic fragrance-free
dermatologist tested
allergy tested
50 mL ℮ 1.7 FL. OZ.
| HYDRABOOST OIL-FREE SUNSCREEN SPF 40
homosalate, octisalate, and avobenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bluemercury (097435361) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogenesis, Inc. | 069117328 | manufacture(72203-015) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.