90081-009 Groovy Grape Hand sanitizer
Hand sanitizer by
Drug Labeling and Warnings
Hand sanitizer by is a Otc medication manufactured, distributed, or labeled by FLTR, INC., Huizhou Baozi Biotechnology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HAND SANITIZER- fltr gelÂ
FLTR, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
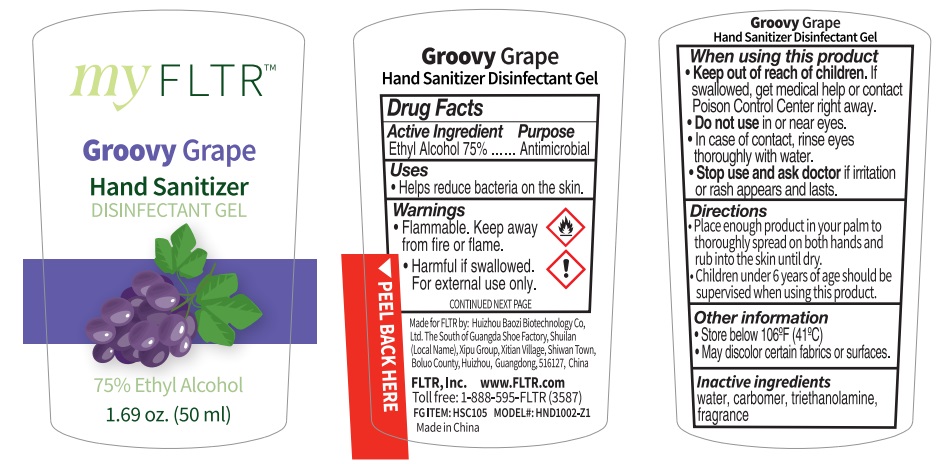
90081-009
Groovy Grape Hand sanitizer
Warnings
Flammable. Keep away from fire or flame.
Harmful if swallowed.
For external use only.
CONTINUED NEXT PAGE
WHEN USING THIS PRODUCT
Keep out of reach of children . If swallowed, get medkal help or contact Poison Control Center right away.
Do not use in or near eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask aoctor if irritatioin or rash appears and lasts.
Keep out of reach of children . If swallowed, get medkal help or contact Poison Control Center right away.
| HAND SANITIZERÂ
fltr gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler -Â FLTR, INC. (117468331) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| FLTR, INC. | 117468331 | label(90081-009) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Huizhou Baozi Biotechnology Co., Ltd. | 419304583 | manufacture(90081-009) | |
Trademark Results [Hand sanitizer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HAND SANITIZER 88958909 not registered Live/Pending |
MAISON BLANCHE, LLC 2020-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.