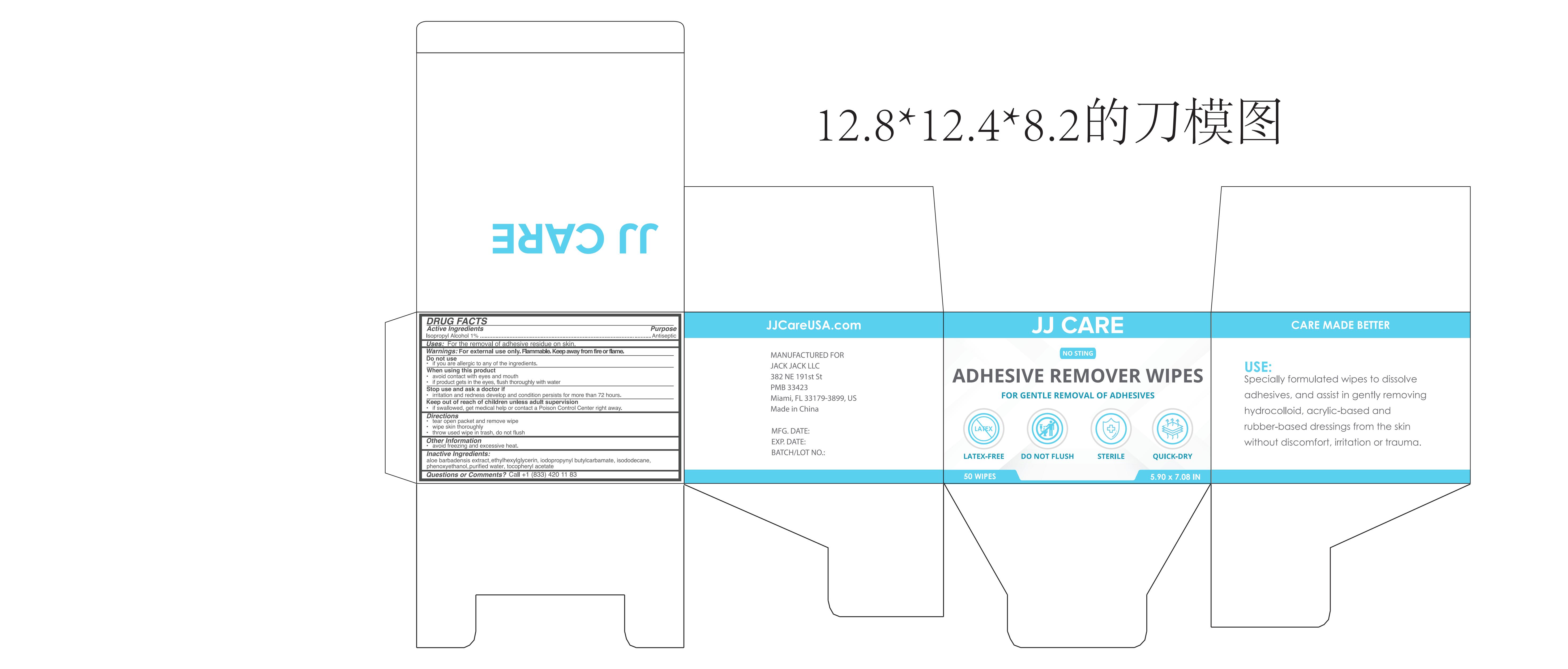
Adhesive Remover Wipes by Ningbo Riway Daily Commodity Co., Ltd Adhesive Remover Wipes
Adhesive Remover Wipes by
Drug Labeling and Warnings
Adhesive Remover Wipes by is a Otc medication manufactured, distributed, or labeled by Ningbo Riway Daily Commodity Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ADHESIVE REMOVER WIPES- isopropyl alcohol cloth
Ningbo Riway Industrial Co., Ltd.
----------
Adhesive Remover Wipes
When using this product,avoid contact with eyes and mouth. If product gets in the eyes,flush thoroughly with water.
Discontinue use and contact a doctor if irritation and redness develop and condition persists for more than 72 hours.
Keep out of reach of children unless under adult supervision. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Tear open packet and remove wipe.
Wipe the skin thoroughly.
Throw used wipe in trash. Do not flush.
| ADHESIVE REMOVER WIPES
isopropyl alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Ningbo Riway Industrial Co., Ltd. (540997562) |
| Registrant - Ningbo Riway Industrial Co., Ltd. (540997562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Riway Industrial Co., Ltd. | 540997562 | manufacture(77267-006) | |
Revised: 12/2025
Document Id: 472666bc-1c14-d03d-e063-6394a90a9ed6
Set id: b1a9c2ab-6761-ebe1-e053-2995a90aa338
Version: 4
Effective Time: 20251230