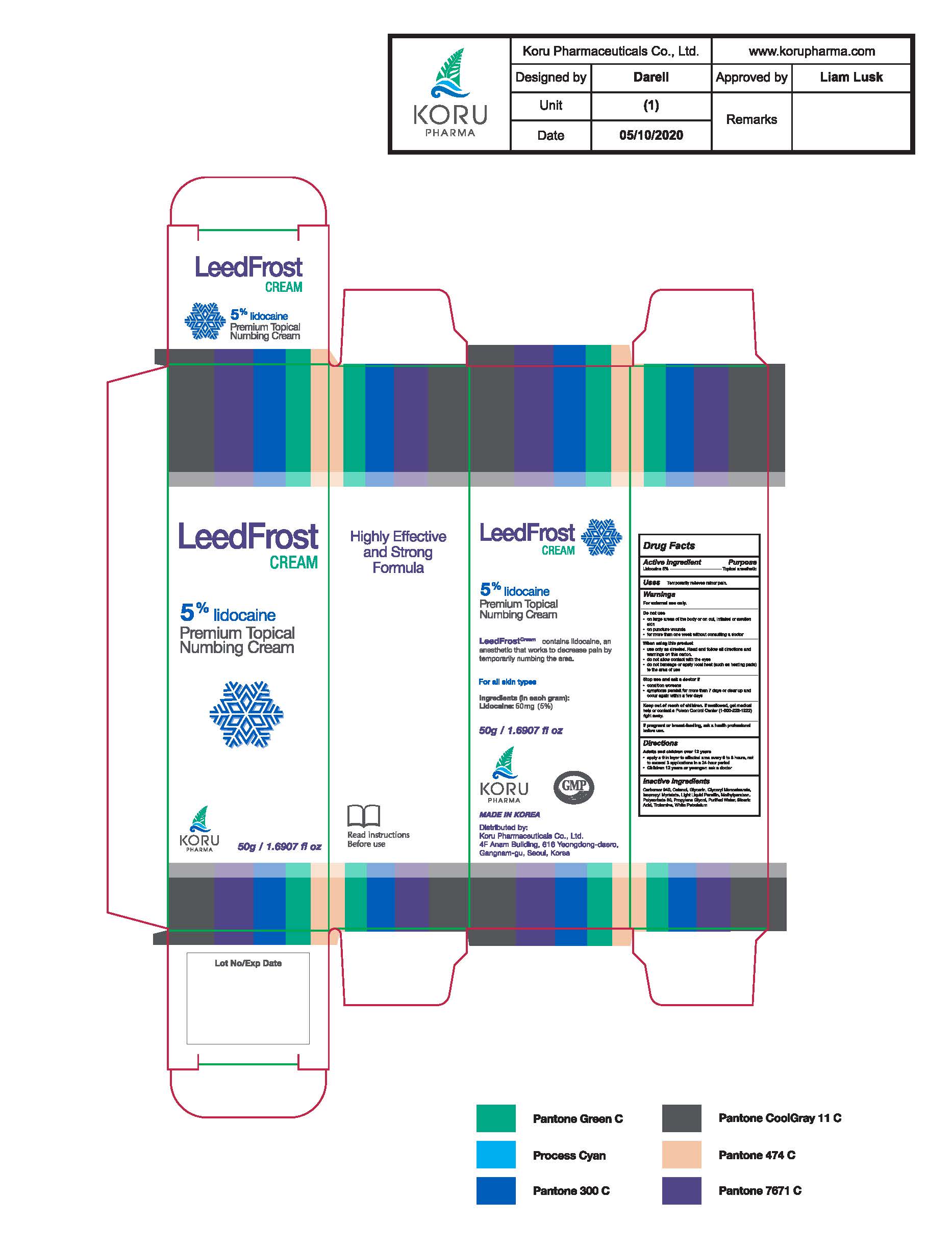
LeedFrost Cream (lidocaine 5%)
LeedFrost Cream (lidocaine 5%) by
Drug Labeling and Warnings
LeedFrost Cream (lidocaine 5%) by is a Otc medication manufactured, distributed, or labeled by Koru Pharmaceuticals Co., Ltd., Koru Pharmaceutical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEEDFROST CREAM (LIDOCAINE 5%)- lidocaine cream
Koru Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LeedFrost Cream (lidocaine 5%)
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
- use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contact with tey eyes
- do not bandage or apply local heat (such as heating pads) to the area of use
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
Adults and children over 12 years
- apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 applications in a 24-hour period
- Children 12 years or younger: ask a doctor
| LEEDFROST CREAM (LIDOCAINE 5%)
lidocaine cream |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Koru Pharmaceuticals (694788172) |
Revised: 10/2021
Document Id: cf59e64c-7747-e3fe-e053-2a95a90a8640
Set id: b1b700e3-b320-7809-e053-2995a90a365b
Version: 2
Effective Time: 20211021
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.