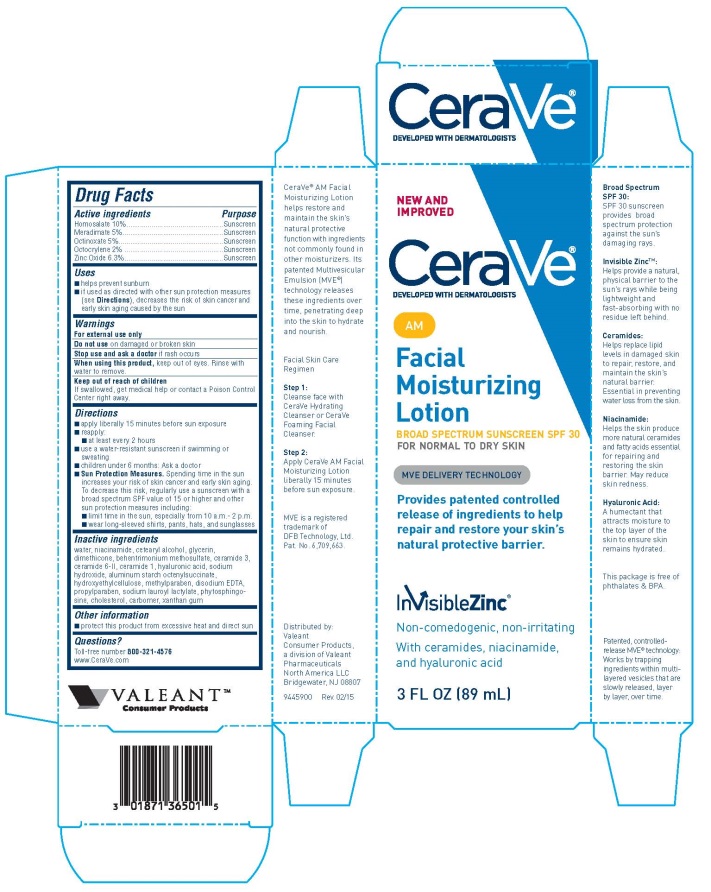
CERAVE AM BROAD SPECTRUM SUNSCREEN SPF 30- homosalate, meradimate, octinoxate, zinc oxide, and octocrylene lotion
CeraVe by
Drug Labeling and Warnings
CeraVe by is a Otc medication manufactured, distributed, or labeled by Valeant Pharmaceuticals North America LLC, Ei INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- ■ apply liberally 15 minutes before sun exposure
- ■ reapply:
- ■ at least every 2 hours
- ■ use a water-resistant sunscreen if swimming or sweating
- ■ children under 6 months: Ask a doctor
- ■Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early
- skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15
- or higher and other sun protection measures including:
- ■ limit time in the sun, especially from 10 a.m.- 2 p.m.
- ■ wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients
water, niacinamide, cetearyl alcohol, glycerin, dimethicone, behentrimonium methosulfate, ceramide 3,
ceramide 6-II, ceramide 1, hyaluronic acid, sodium hydroxide, aluminum starch octenylsuccinate,
hydroxyethylcellulose, methylparaben, disodium EDTA, propylparaben, sodium lauroyl lactylate, phytosphingosine, cholesterol, carbomer, xanthan gum
- Otherinformation
- Questions?
-
PRINCIPAL DISPLAY PANEL - 3 FL OZ Bottle
NEW AND
IMPROVED
CeraVe®
DEVELOPED WITH DERMATOLOGISTSAM
Facial
Moisturizing
Lotion
BROAD SPECTRUM SUNSCREEN SPF 30
FOR NORMAL TO DRY SKIN
MVE DELIVERY TECHNOLOGY
Provides patented controlled
release of ingredients to help
repair and restore your skin's
natural protective barrier.
InvisibleZinc®
Non-comedogenic, non-irritating
With ceramides, niacinamide,
and hyaluronic acid
3 FL OZ (89 mL)
-
INGREDIENTS AND APPEARANCE
CERAVE AM BROAD SPECTRUM SUNSCREEN SPF 30
homosalate, meradimate, octinoxate, zinc oxide, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0187-1365 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 mL MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 50 mg in 1 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 20 mg in 1 mL Zinc oxide (UNII: SOI2LOH54Z) (Zinc oxide - UNII:SOI2LOH54Z) Zinc oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONIC ACID (UNII: S270N0TRQY) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-1365-01 89 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 06/15/2015 Labeler - Valeant Pharmaceuticals North America LLC (042230623) Establishment Name Address ID/FEI Business Operations Ei INC. 105803274 MANUFACTURE(0187-1365)
Trademark Results [CeraVe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERAVE 98843091 not registered Live/Pending |
Yan Run 2024-11-08 |
 CERAVE 98765114 not registered Live/Pending |
Du, Ting 2024-09-23 |
 CERAVE 98733953 not registered Live/Pending |
Haijiang Wang 2024-09-05 |
 CERAVE 98428743 not registered Live/Pending |
Zheng, XiaoLi 2024-03-01 |
 CERAVE 97255760 not registered Live/Pending |
L'Oreal USA Creative, Inc. 2022-02-07 |
 CERAVE 78519354 3234519 Live/Registered |
L'Oreal USA Creative, Inc. 2004-11-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.