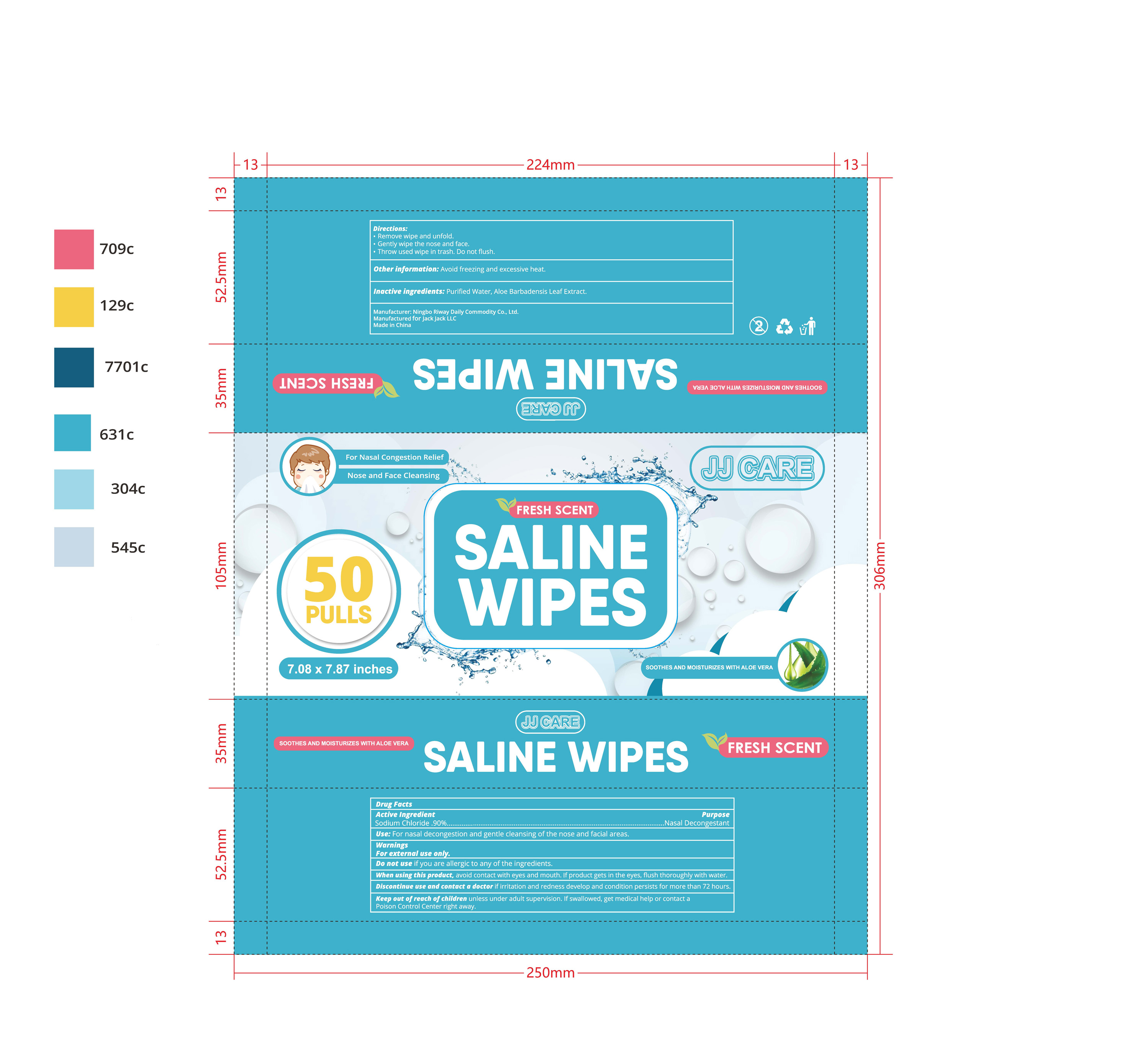
Saline Wipes by Ningbo Riway Daily Commodity Co., Ltd
Saline Wipes by
Drug Labeling and Warnings
Saline Wipes by is a Otc medication manufactured, distributed, or labeled by Ningbo Riway Daily Commodity Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SALINE WIPES- sodium chloride cloth
Ningbo Riway Daily Commodity Co., Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
When using this product,avoid contact with eyes and mouth. If product gets in the eyes,flush thoroughly with water.
Discontinue use and contact a doctor if irritation and redness develop and condition persists for more than 72 hours.
Keep out of reach of children unless under adult supervision. If swallowed, get medical help or contact a Poison Control Center right away.
| SALINE WIPES
sodium chloride cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ningbo Riway Daily Commodity Co., Ltd (540997562) |
| Registrant - Ningbo Riway Daily Commodity Co., Ltd (540997562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Riway Daily Commodity Co., Ltd | 540997562 | manufacture(77267-007) | |