Always Foundation Makeup Broad Spectrum SPF 15 Bisque
Always Foundation Makeup Broad Spectrum SPF 15 Bisque by
Drug Labeling and Warnings
Always Foundation Makeup Broad Spectrum SPF 15 Bisque by is a Otc medication manufactured, distributed, or labeled by JAFRA COSMETICS INTERNATIONAL, Jafra Manufacturing, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALWAYS FOUNDATION MAKEUP BROAD SPECTRUM SPF 15 BISQUE- homosalate, octisalate, zinc oxide liquid
JAFRA COSMETICS INTERNATIONAL
----------
Always Foundation Makeup Broad Spectrum SPF 15 Bisque
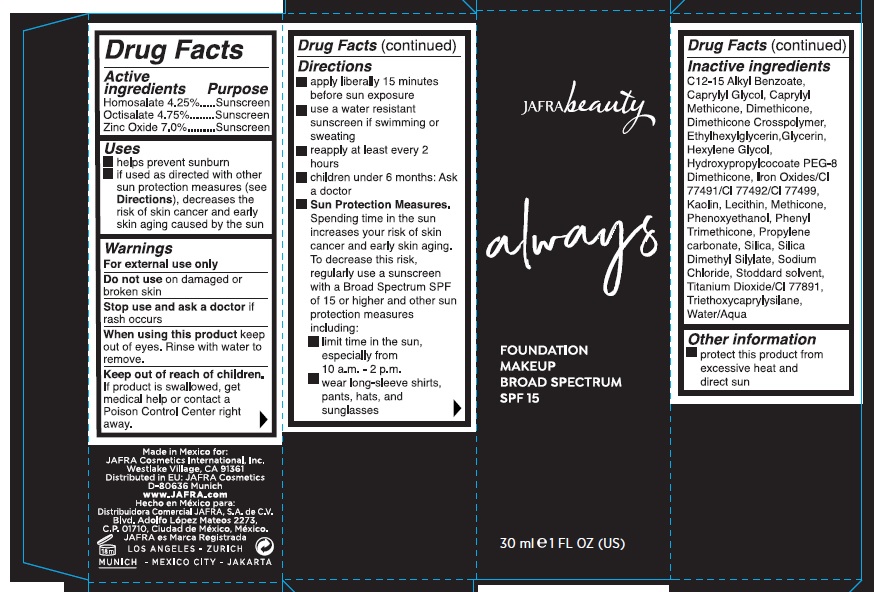
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not useon damaged or broken skin
Stop use and ask a doctorif rash occurs
When using this productkeep out of eyes. Rinse with water to remove.
Keep out of reach of children.If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months: Ask a doctor
Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients.
C12-15 Alkyl Benzoate, Caprylyl Glycol, Caprylyl Methicone, Dimethicone, Dimethicone Crosspolymer, Ethylhexylglycerin, Glycerin, Hexylene Glycol, Hydroxypropylcocoate PEG-8 Dimethicone, Iron Oxides/CI 77491/CI 77492/CI 77499, Kaolin, Lecithin, Methicone, Phenoxyethanol, Phenyl Trimethicone, Propylene Carbonate, Silica, Silica Dimethyl Silylate, Sodium Chloride, Stoddard Solvent, Titanium Dioxide/CI 77891, Triethoxycaprylylsilane, Water/Aqua
| ALWAYS FOUNDATION MAKEUP BROAD SPECTRUM SPF 15 BISQUE
homosalate, octisalate, zinc oxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - JAFRA COSMETICS INTERNATIONAL (041676479) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Distribuidora Comercial Jafra, S.A. de C.V. | 951612777 | manufacture(68828-714) | |