MICLARA LQ- triprolidine hydrochloride liquid
Miclara LQ by
Drug Labeling and Warnings
Miclara LQ by is a Otc medication manufactured, distributed, or labeled by Key Therapeutics, TG United. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
Miclara LQ- doxylamine succinate, pseudoephedrine hydrochloride liquid
Key Therapeutics
Disclaimer: Most OTC drugs are not reviewed and approved by FDA; however, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
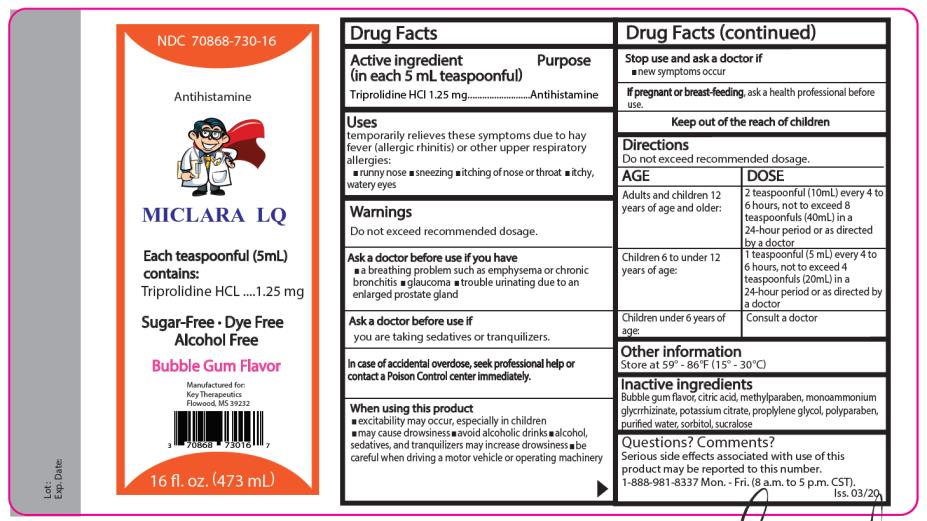
- MICLARA LQ LIQUID
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not exceed recommended dosage.
Do not use this product if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- may cause excitability especially in children
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- be careful when driving a motor vehicle or operating machinery
- a breathing problem such as emphysema or chronic bronchitis
-
Directions
Do not exceed recommended dosage.
Adults and children
12 years of age and older:
Children 6 to under 12 years of age:
2 teaspoonfuls (10 mL) every 4 to 6 hours, not to exceed
8 teaspoonfuls (40mL) in 24-hour
period or as directed by a doctor.
1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 4 teaspoonfuls (20mL) in a 24-hour period or as directed by a doctor.
Children under 6 years of age: Consult a doctor
- Other information
- Inactive ingredients
- Questions? Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MICLARA LQ
triprolidine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70868-730 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 1.25 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70868-730-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 05/15/2020 Labeler - Key Therapeutics (080318791)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.