DERMATOOL® FUNGAL NAIL SOLUTION
DERMATOOL Fungal Nail Solution by
Drug Labeling and Warnings
DERMATOOL Fungal Nail Solution by is a Otc medication manufactured, distributed, or labeled by RENU LABORATORIES, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DERMATOOL FUNGAL NAIL SOLUTION- tolnaftate oil
RENU LABORATORIES, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DERMATOOL® FUNGAL NAIL SOLUTION
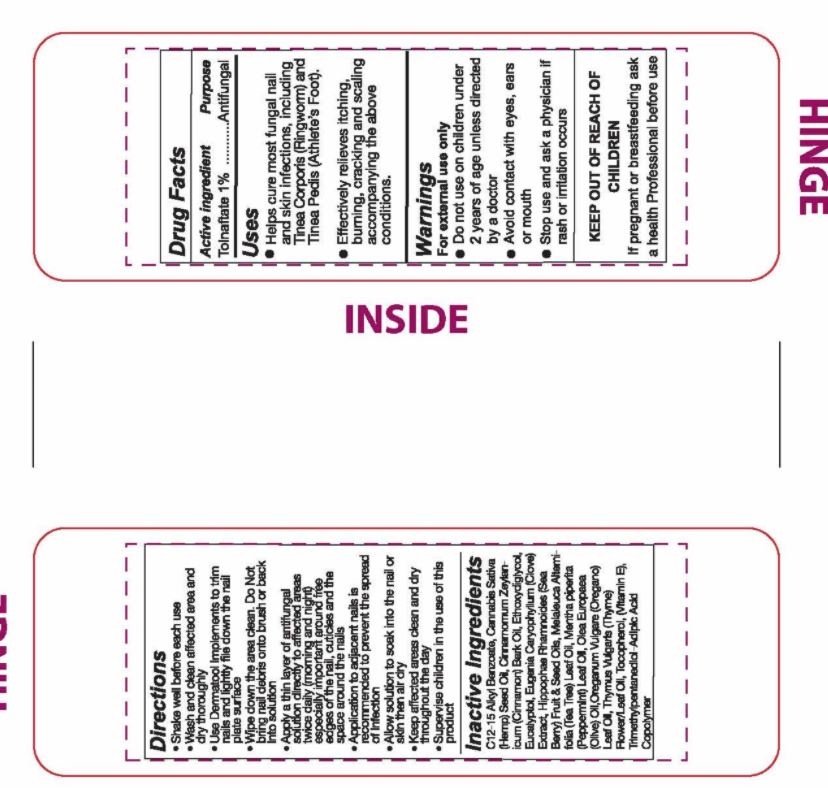
Uses
- Helps cure most fungal nail and skin infections, including Tinea Corporis (Ringworm) and Tinea Pedis (Athlete's Foot).
- Effectively relieves itching, burning, cracking and scaling accompanying the above conditions.
Warnings
For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- Avoid contact with eyes, ears or mouth
- Stop use and ask a physician if rash or irritation occurs
Directions
- Shake well before each use
- Wash and clean affected area and dry thoroughly
- Use Dermatool implements to trim nails and lightly file down the nail plate surface
- Wipe down the area clean. Do not bring nail debris onto brush or back into solution
- Apply a thin layer of antifungal solution directly to affected areas twice daily (morning and night) especially important around the edges of the nail, cuticles and the space around the nails
- Application to adjacent nails is recommended to prevent the spread of infection
- Allow solution to soak into the nail or skin then air dry
- Keep affected areas clean and dry throughout the day
- Supervise children in the use of this product.
Inactive Ingredients
C12-15 Alkyl Benzoate, Cannabis Sativa (Hemp) Seed Oil, Cinnamomum Zeylanicum (Cinnamon) Bark Oil, Ethoxydiglycol, Eucalyptol, Eugenia Caryophyllum (Clove) Extract, Hippophae Rhamnoides (Sea Berry) Fruit & Seed Oils, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Mentha piperita (Peppermint) Oil, Olea Europaea (Olive) Oil, Oreganum Vulgare (Oregano) Leaf Oil, Thymus Vulgaris (Thyme) Flower/Leaf Oil, Tocopherol (Vitamin E), Trimethylpentanediol-Adipic Acid Copolymer
| DERMATOOL FUNGAL NAIL SOLUTION
tolnaftate oil |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - RENU LABORATORIES, INC. (945739449) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| RENU LABORATORIES, INC. | 945739449 | manufacture(76348-584) | |