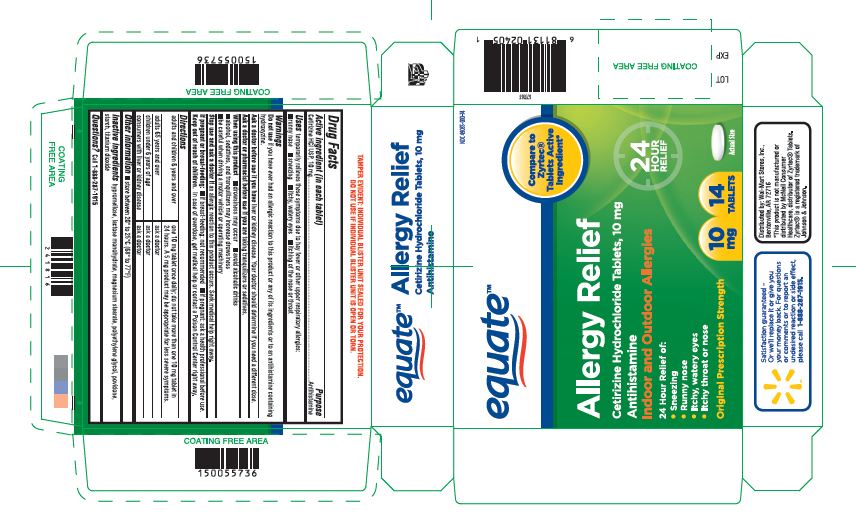
Cetirizine Hydrochloride Tablets
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Wal-Mart Stores Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALLERGY RELIEF- cetirizine hydrochloride tablets tablet, film coated
Wal-Mart Stores Inc
----------
Cetirizine Hydrochloride Tablets
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
.
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistaminecontaining hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Directions
| adults and children 6 years and over | one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
| adults 65 years and over | ask a doctor |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
| ALLERGY RELIEF
cetirizine hydrochloride tablets tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Wal-Mart Stores Inc (051957769) |
Revised: 12/2019
Document Id: 7c72e74f-c713-feb3-d4cc-d90e1320d76c
Set id: b2270eaf-d62b-392c-ee48-bc8563c3efc5
Version: 10
Effective Time: 20191218
Wal-Mart Stores Inc