Skin delight Hand WashGel by INTERKOS CO., LTD. Drug Facts
Skin delight Hand WashGel by
Drug Labeling and Warnings
Skin delight Hand WashGel by is a Otc medication manufactured, distributed, or labeled by INTERKOS CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SKIN DELIGHT HAND WASHGEL- alcohol gel
INTERKOS CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Water
Carbomer
Triethanolamine
PEG-60 Hydrogenated Castor Oil
Fragrance
Chlorphenesin
Trideceth-10
Butylene Glycol
Salix Alba (Willow) Bark Extract
Origanum Vulgare Leaf Extract
Chamaecyparis Obtusa Leaf Extract
Portulaca Oleracea Extract
Lactobacillus/Soybean Ferment Extract
Cinnamomum Cassia Bark Extract
Scutellaria Baicalensis Root Extract
Vincetoxicum Atratum Extract
1,2-Hexanediol
Caprylyl Glycol
Aloe Barbadensis Leaf Extract
Illicium Verum (Anise) Fruit Extract
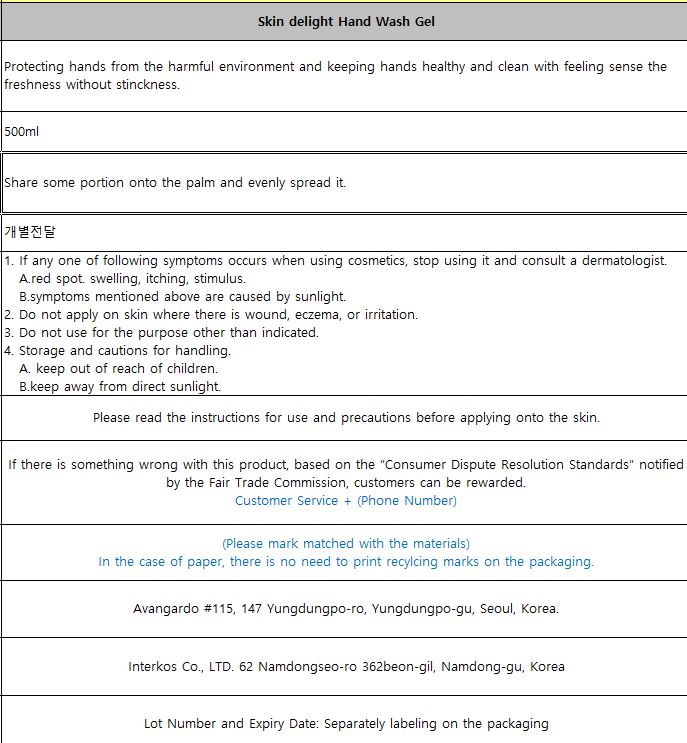
Protecting hands from the harmful environment and keeping hands healthy and clean with feeling sense the freshness without stinckness.
1. If any one of following symptoms occurs when using cosmetics, stop using it and consult a dermatologist.
A.red spot. swelling, itching, stimulus.
B.symptoms mentioned above are caused by sunlight.
2. Do not apply on skin where there is wound, eczema, or irritation.
3. Do not use for the purpose other than indicated.
4. Storage and cautions for handling.
A. keep out of reach of children.
B.keep away from direct sunlight.
| SKIN DELIGHT HAND WASHGEL
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - INTERKOS CO., LTD. (689850314) |
| Registrant - INTERKOS CO., LTD. (689850314) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| INTERKOS CO., LTD. | 689850314 | manufacture(77049-0006) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.