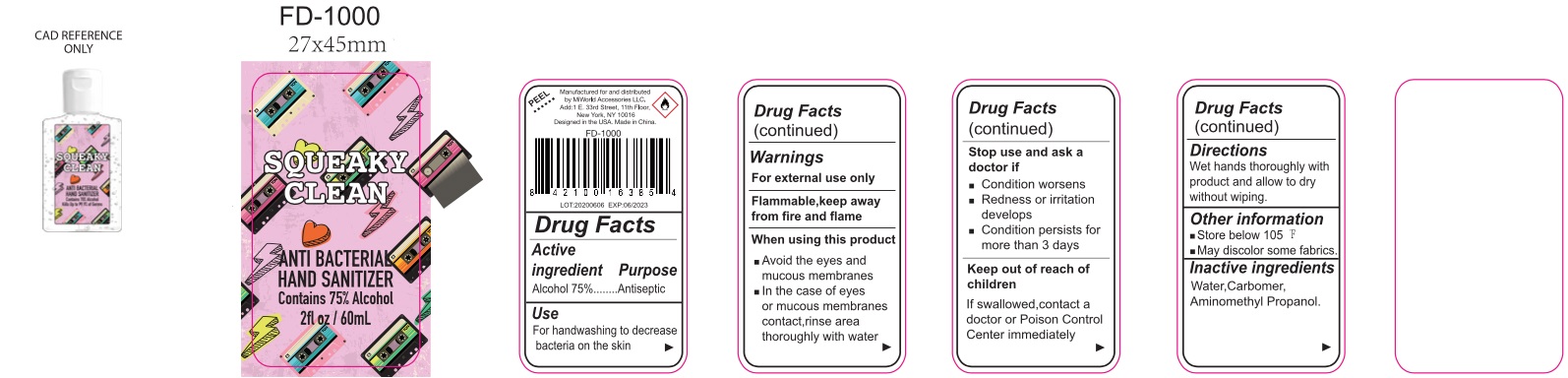
Squeaky Clean Antibacterial Hand Sanitizer
Squeaky Clean Antibacterial Hand Sanitizer by
Drug Labeling and Warnings
Squeaky Clean Antibacterial Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by MiWorld Accessories LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SQUEAKY CLEAN ANTIBACTERIAL HAND SANITIZER- alcohol gel
MiWorld Accessories LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Squeaky Clean Antibacterial Hand Sanitizer
Warnings
For external use only
Flammable, keep away from fire and flame
When using this product
- Avoid the eyes and mucous membranes
- In case of eyes or mucous membranes contact, rinse area thoroughly with water
| SQUEAKY CLEAN ANTIBACTERIAL HAND SANITIZER
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MiWorld Accessories LLC (078708128) |
Revised: 12/2022
Document Id: f049eb75-5efc-5caa-e053-2a95a90a804c
Set id: b25ab57c-2777-4592-e053-2995a90a991b
Version: 2
Effective Time: 20221220