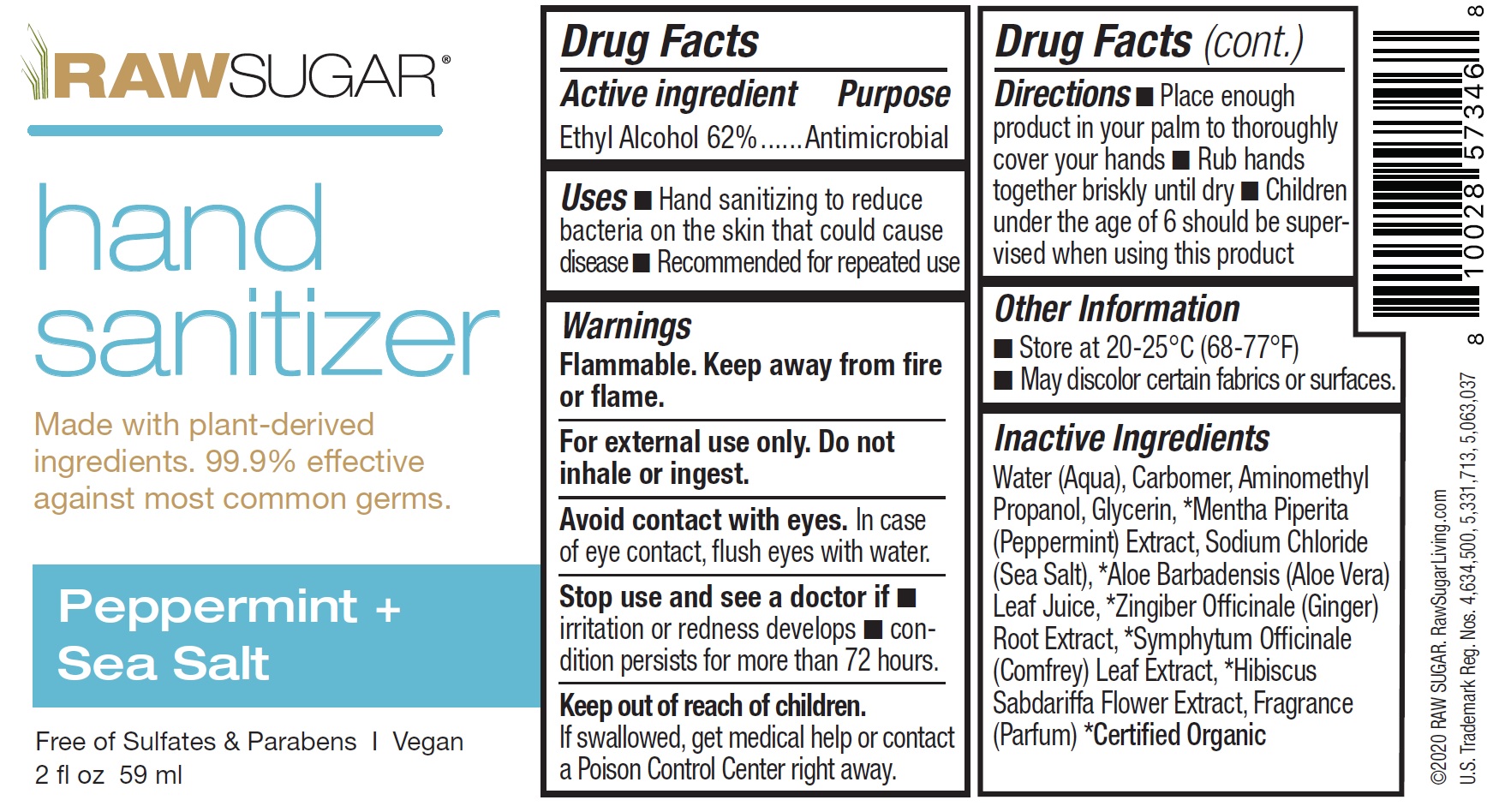
Raw Sugar Hand Sanitizer, Peppermint and Sea Salt
Raw Sugar Hand Sanitizer Peppermint and Sea Salt by
Drug Labeling and Warnings
Raw Sugar Hand Sanitizer Peppermint and Sea Salt by is a Otc medication manufactured, distributed, or labeled by Bolero Home Decor, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RAW SUGAR HAND SANITIZER PEPPERMINT AND SEA SALT- alcohol gel
Bolero Home Decor, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Raw Sugar Hand Sanitizer, Peppermint and Sea Salt
Uses
- Hand sanitizing to reduce bacteria on the skin that could cause disease
- Recommended for repeated use
Warnings
Flammable. Keep away from fire or flame.
For external use only. Do not inhale or ingest.
Avoid contact with eyes. In case of eye contact, fush eyes with water.
Directions
- Place enough product in your palm to thoroughly cover your hands
- Rub hands together briskly until dry
- Children under the age of 6 should be supervised when using this product
Inactive Ingredients
Water (Aqua), Carbomer, Aminomethyl Propanol, Glycerin, *Mentha Piperita (Peppermint) Extract, Sodium Chloride (Sea Salt), *Aloe Barbadensis (Aloe Vera) Leaf Juice, *Zingiber Offfcinale (Ginger) Root Extract, *Symphytum Offcinale (Comfrey) Leaf Extract, *Hibiscus Sabdariffa Flower Extract, Fragrance (Parfum)) *Certifed Organic
| RAW SUGAR HAND SANITIZER PEPPERMINT AND SEA SALT
alcohol gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Bolero Home Decor, Inc. (067967365) |