STERILE WATER FOR INJECTION
Sterile Water by
Drug Labeling and Warnings
Sterile Water by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Company, Baxter Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
STERILE WATER- water injection, solution
Baxter Healthcare Company
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
STERILE WATER FOR INJECTION
Health Care Professional Letter
Reporting Adverse Events or Product Quality Issues
To report adverse events associated with these imported products, please call Baxter at 1-866-888-2472, or fax: 1-800-759-1801. Adverse events or quality problems experienced with the use of these imported products may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
To report product quality issues associated with these imported products, please contact Baxter Product Surveillance through Baxter Product Feedback Portal (https://productfeedback.baxter.com)
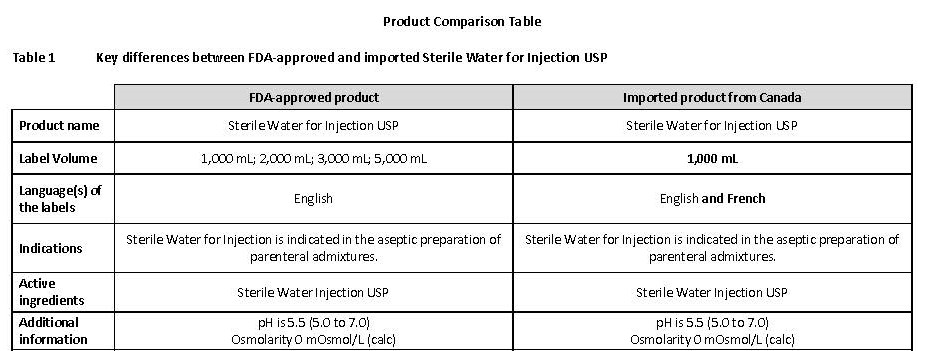
Please refer to the FDA-approved prescribing information for each drug product listed below:
Sterile Water for Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018632s051lbl.pdf)
70% Dextrose Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020047s021lbl.pdf)
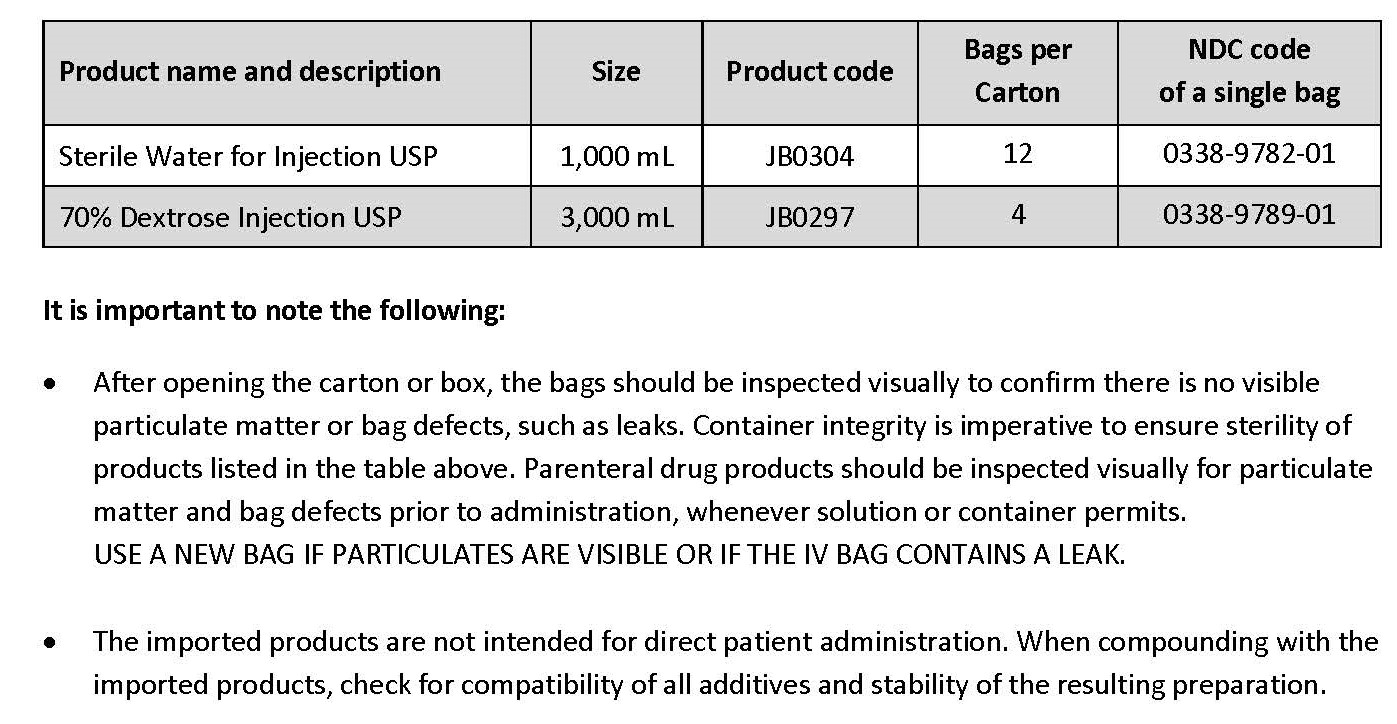
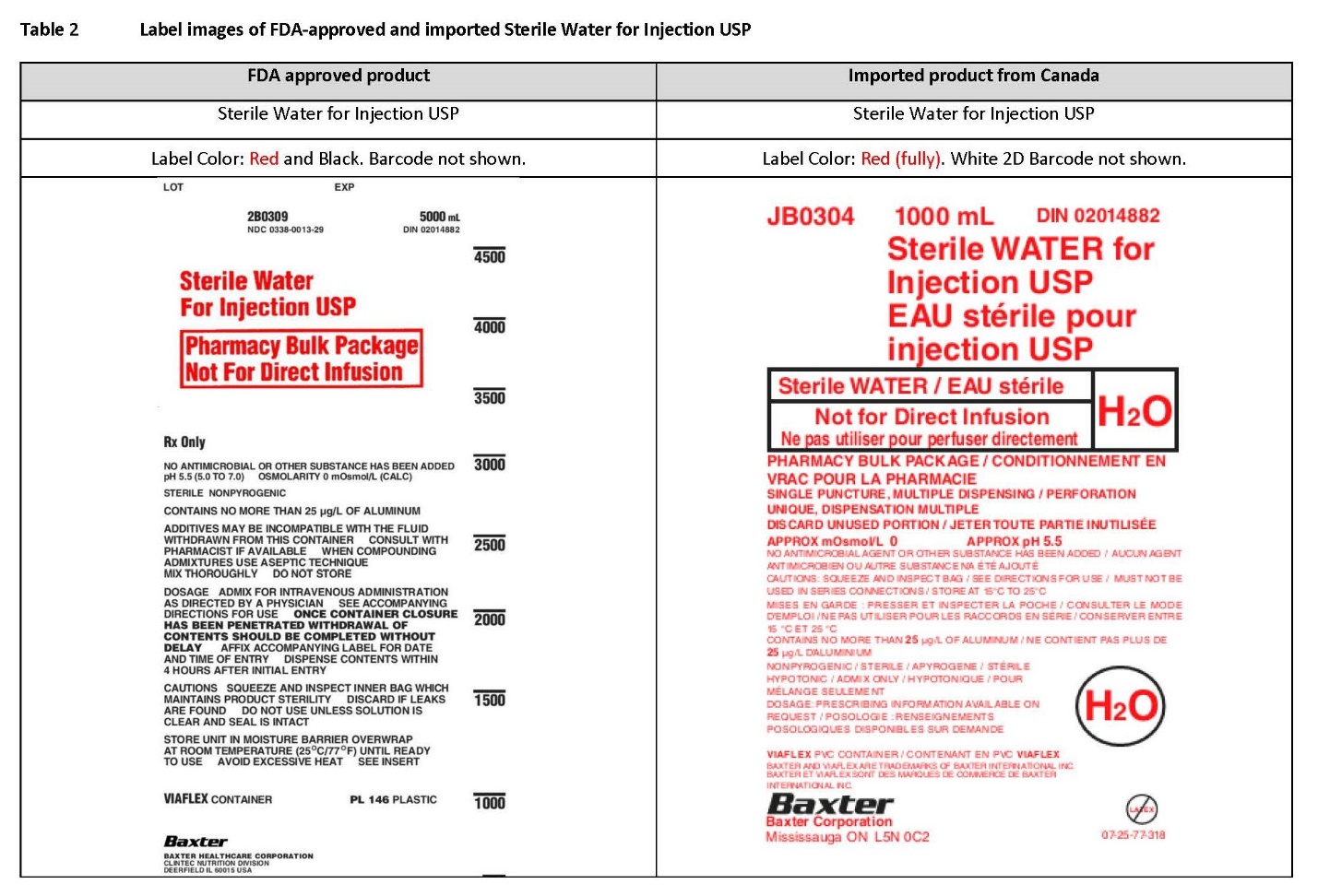
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
JB0304 1000 mL DIN 02014882
Sterile WATER for
Injection USP
EAU stérile pour
injection USP
Sterile WATER / EAU stérile
Not for Direct Infusion
Ne pas utilizer pour perfuser directment
H2O
PHARMACY BULK PACKAGE / CONDITIONNEMENT EN
VRAC POUR LA PHARMACIE
SINGLE PUNCTURE, MULTIPLE DISPENSING / PERFORATION
UNIQUE, DISPENSATION MULTIPLE
DISCARD UNUSED PORTION / JETER TOUTE PARTIE INUTILISÉE
APPROX mOsmol/L 0 APPROX pH 5.5
NO ANTIMICROBIAL AGENT OR OTHER SUBSTANCE HAS BEEN ADDED / AUCUN AGENT
ANTIMICROBIEN OU AUTRE SUBSTANCE NA ÉTÉ AJOUTÉ
CAUTIONS: SQUEEZE AND INSPECT BAG / SEE DIRECTIONS FOR USE / MUST NOT BE
USED IN SERIES CONNECTIONS / STORE AT 15°C ET 25°C
MISES EN GARD : PRESSER ET INSPECTOR LA POCHE / CONSULTER LE MODE
D’ÉMPLOI / NE PAS UTILISER POUR LES RACCORDS EN SÉRIE / CONSERVER ENTRE
15°C ET 25°C
CONTAINS NO MORE THAN 25 µg/L OF ALUMINUM / NE CONTINENT PAS PLUS DE
25 µg/L D’ALUMINIUM
NONPYROGENIC / STERILE / APYROGENE / STÉRILE
HYPOTONIC / ADMIX ONLY / HYPTONIQUE / POUR
MÉLANGE SUELEMENT
DOSAGE: PRESCRIBING INFORMATION AVAILABLE ON
REQUEST / POSOLOGIE : RENSEIGNEMENTS
POSOLOGIQUES DISPONIBLES SUR DEMANDE
H2O Label
VIAFLEX PVC CONTAINER / CONTENANT EN PVC VIAFLEX
BAXTER AND VIAFLEX TRADEMARKS OF BAXTER INTERNATIONAL, INC.
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL, INC.
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No Latex Label
07-25-77-318
JB-03-04
12 – 1000 mL units
Store at 15°C to 25°C
Sterile Water for Injection USP
Lot: WWWWWWWWW EXP: 2099 99
2DBarcode
(01)20809080000528
Pharmacy Bulk
| STERILE WATER
water injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Company (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Corporation | 205087968 | ANALYSIS(0338-9782) , LABEL(0338-9782) , MANUFACTURE(0338-9782) , STERILIZE(0338-9782) , PACK(0338-9782) | |