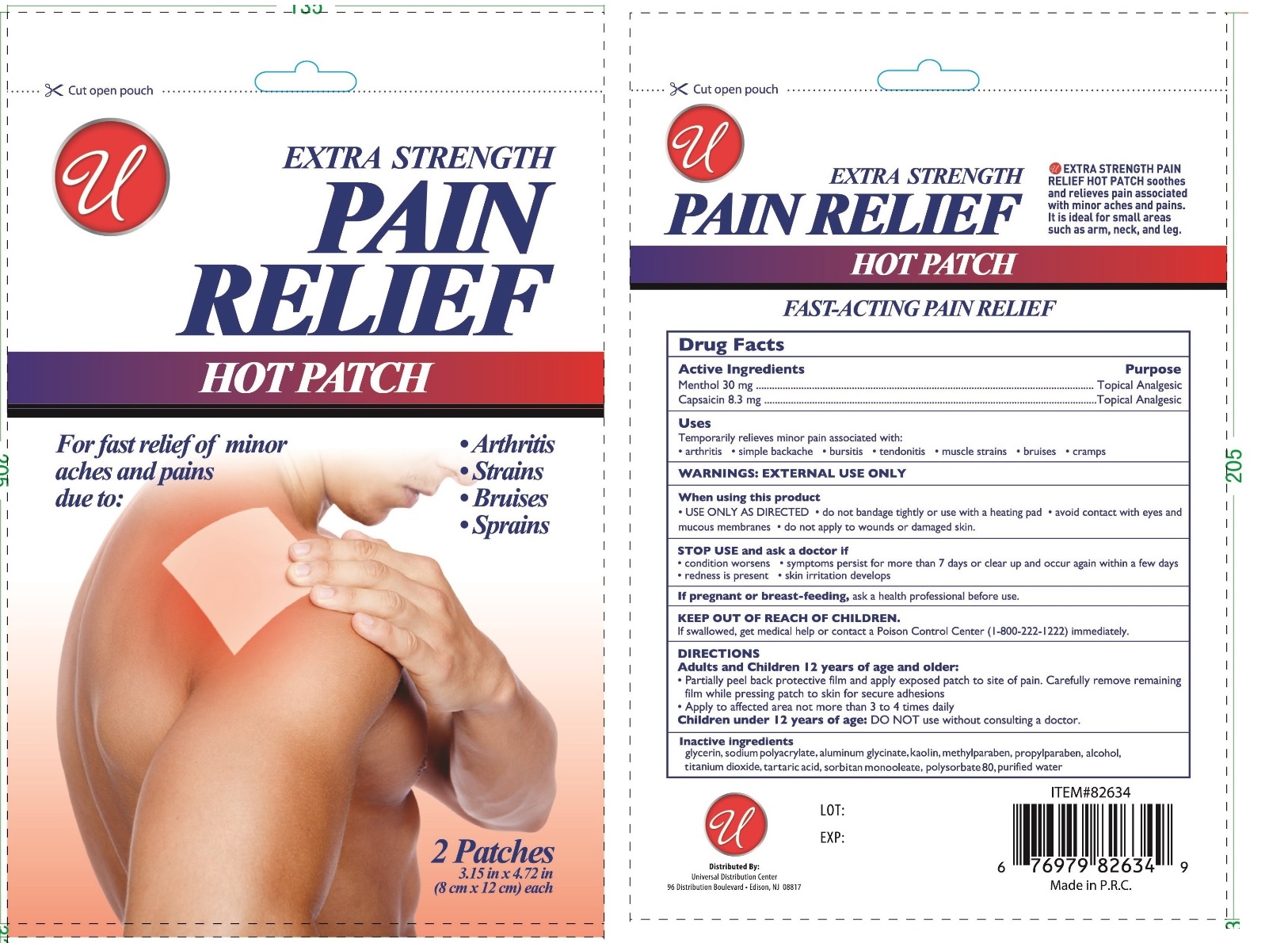
Pain Relief Hot Patch by Universal Distribution Center LLC / Beijing HKKY Medical Tech. Co., Ltd. Pain Relief Hot Patch
Pain Relief Hot Patch by
Drug Labeling and Warnings
Pain Relief Hot Patch by is a Otc medication manufactured, distributed, or labeled by Universal Distribution Center LLC, Beijing HKKY Medical Tech. Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEF HOT PATCH- menthol and capsaicin patch
Universal Distribution Center LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pain Relief Hot Patch
Uses
Temporarily relieves minor pains associated with:
- arthritis simple backache bursitis tendonitis muscle strains bruises cramps
WARNINGS:
EXTERNAL USE ONLY
When using this product
USE ONLY AS DIRECTED do not bandage tightly or use with a heating pad avoid contact with eyes and mucous membranes do not apply to wounds or damaged skin.
STOP USE and ask a doctor if
condition worsens symptoms persist for more than 7 days or clear up and occur again within a few days
redness is present skin irritation develops
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediately.
DIRECTIONS
Adults and Children 12 years of age and older:
- Partially peel back protective film and apply exposed patch to site of pain. Carefully remove remaining film while pressing patch to skin for secure adhesions
- Apply to affected area not more than 3 to 4 times a daily
Children under 12 years of age: DO NOT use without consulting a doctor.
Inactive Ingredients
glycerin, sodium polyacrylate, aluminum glycinate, kaolin, methylparaben, propylparaben, alcohol, titanium dioxide, tartaric acid, sorbitan monooleate, polysorbate 80, purified water
| PAIN RELIEF HOT PATCH
menthol and capsaicin patch |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Universal Distribution Center LLC (019180459) |
| Registrant - Universal Distribution Center LLC (019180459) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Dingtai Pharmaceutical Co., Ltd | 420598724 | manufacture(52000-034) | |