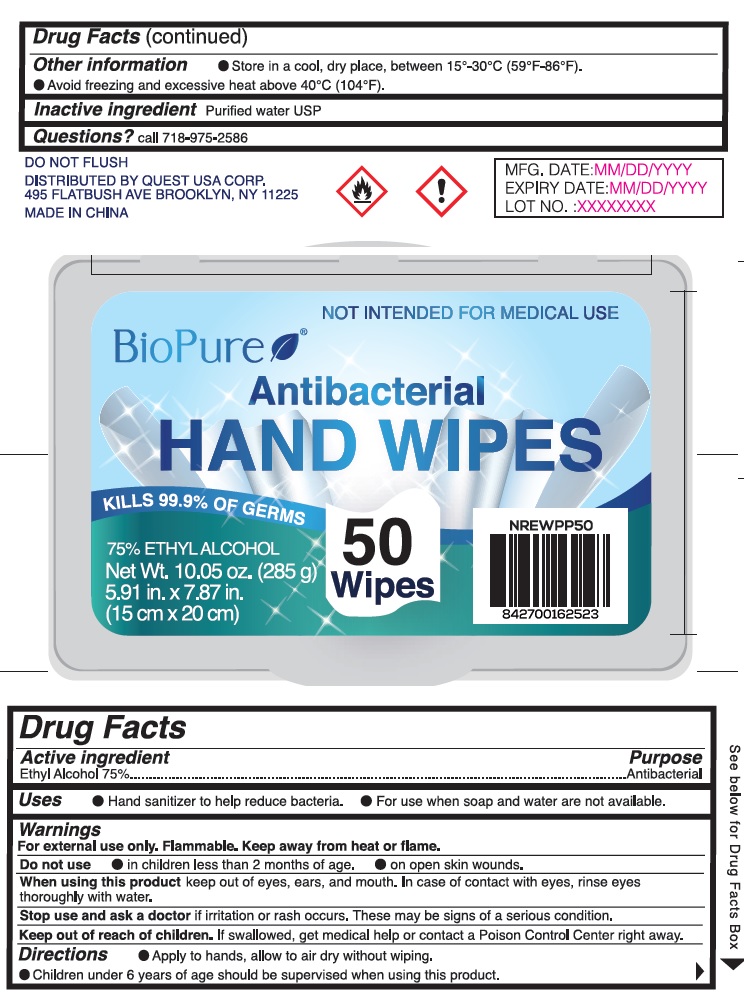
BioPure Antibacterial Hand Wipes, 75% Ethyl Alcohol
BioPure Antibacterial Hand Wipes by
Drug Labeling and Warnings
BioPure Antibacterial Hand Wipes by is a Otc medication manufactured, distributed, or labeled by QUEST USA CORP.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIOPURE ANTIBACTERIAL HAND WIPES- alcohol cloth
QUEST USA CORP.
----------
BioPure Antibacterial Hand Wipes, 75% Ethyl Alcohol
Warnings
For external use only. Flammable. Keep away from heat or flame.
Directions
- Apply to hands, allow to air dry without wiping.
- Children under 6 years of age should be supervised when using this product.
| BIOPURE ANTIBACTERIAL HAND WIPES
alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - QUEST USA CORP. (079869689) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang iColor Biotech CO.,LTD | 554528308 | manufacture(78691-019) | |
Revised: 2/2024
Document Id: 1134ab8a-4e45-905d-e063-6394a90ac34b
Set id: b2a911bd-61cd-495b-e053-2995a90a2211
Version: 4
Effective Time: 20240212
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.