BIOMOX- amoxicillin suspension
Biomox by
Drug Labeling and Warnings
Biomox by is a Animal medication manufactured, distributed, or labeled by Virbac AH, Inc., Medispray Laboratories Private Ltd, Centrient Pharmaceuticals Netherlands, Alcami Missouri Corporation, Alcami Carolinas Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- Inactive Ingredients
-
ACTION
Amoxicillin has bactericidal activity against susceptible organisms similar to that of ampicillin. It acts by inhibiting the biosynthesis of bacterial wall mucopeptides. Most strains of the following gram-positive and gram-negative bacteria have demonstrated susceptibility to amoxicillin, both in vitro and in vivo: nonpenicillinase-producing staphylococci, alpha- and beta-hemolytic streptococci, Streptococcus faecalis, Escherichia coli and Proteus mirabilis. Amoxicillin does not resist destruction by penicillinase; therefore, it is not effective against penicillinase-producing bacteria, particularly resistant staphylococci. Most strains of Enterobacter and Klebsiella and all strains of Pseudomonas are resistant.
Amoxicillin may be given without regard to meals because it is stable in gastric acid. It is rapidly absorbed following oral administration and diffuses readily into most body fluids and tissues. It diffuses poorly into the brain and spinal fluid except when the meninges are inflamed. Most of the amoxicillin is excreted in the urine unchanged.
-
INDICATIONS
BIOMOX® (amoxicillin) for oral suspension is indicated in the treatment of the following infections in dogs when caused by susceptible strains of organisms:
BACTERIAL DERMATITIS due to Staphylococcus aureus, Streptococcus spp., Staphylococcus spp., and E. coli.
SOFT TISSUE INFECTIONS (abscesses, wounds, lacerations) due to Staphylococcus aureus, Streptococcus spp., E. coli, Proteus mirabilis and Staphylococcus spp.
As is true with all antibiotic therapy, appropriate in vitro cultures and sensitivities should be conducted prior to treatment.
- CONTRAINDICATIONS
- ADVERSE REACTIONS
- WARNINGS
- PRECAUTIONS
- CAUTION
- DOSAGE AND ADMINISTRATION
-
DIRECTIONS FOR MIXING ORAL SUSPENSION
Add sufficient water to the bottle as indicated in the table below and shake vigorously. Each mL of suspension will contain 50 mg of amoxicillin as the trihydrate.
Bottle Size Amount of Water to
Add for Reconstitution15 mL 11 mL 30 mL 21 mL Note: When stored at room temperature or in refrigerator, discard unused portion of reconstituted suspension after 14 days.
- SUPPLY
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 15 mL Powder Bottle
NDC-051311-300-15
Virbac
ANIMAL HEALTHBIOMOX®
(amoxicillin)VETERINARY FOR ORAL SUSPENSION
Equivalent to 0.75 g amoxicillin
When reconstituted, concentration is
50 mg/mL amoxicillin as the trihydrateCAUTION: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.NADA # 65-495, Approved by FDA
15 mL (when mixed)

-
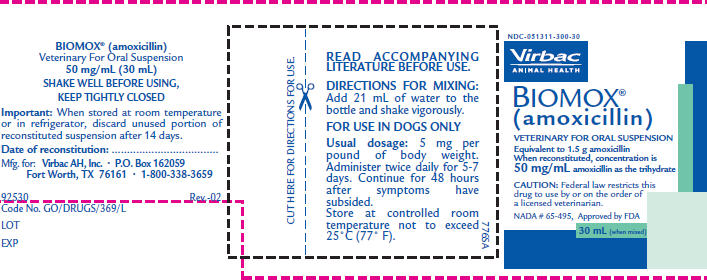
PRINCIPAL DISPLAY PANEL - 30 mL Powder Bottle
NDC-051311-300-30
Virbac
ANIMAL HEALTHBIOMOX®
(amoxicillin)VETERINARY FOR ORAL SUSPENSION
Equivalent to 1.5 g amoxicillin
When reconstituted, concentration is
50 mg/mL amoxicillin as the trihydrateCAUTION: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.NADA # 65-495, Approved by FDA
30 mL (when mixed)
-
INGREDIENTS AND APPEARANCE
BIOMOX
amoxicillin suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 0.75 g in 15 mL Product Characteristics Color WHITE (off-white to pinkish) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-300-15 15 mL in 1 BOTTLE, DROPPER 2 NDC: 51311-300-30 30 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065495 05/24/2010 Labeler - Virbac AH, Inc. (131568396) Establishment Name Address ID/FEI Business Operations Medispray Laboratories Private Ltd 915793457 MANUFACTURE Establishment Name Address ID/FEI Business Operations Teva Pharmaceutical USA, Inc. 611568031 api manufacture
Trademark Results [Biomox]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOMOX 74006481 1686993 Live/Registered |
BIOCRAFT LABORATORIES, INC. 1989-11-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.