Itch Relief Cream EXTRA STRENGTH- diphenhydramine hydrochloride and zinc acetate
Itch Relief by
Drug Labeling and Warnings
Itch Relief by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy, Teva Pharmaceuticals USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ITCH RELIEF EXTRA STRENGTH- diphenhydramine hydrochloride and zinc acetate cream
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Itch Relief Cream EXTRA STRENGTH- diphenhydramine hydrochloride and zinc acetate
USES For the temporary relief of pain and itching associated with
minor burns sunburn minor cuts scrapes insect bites minor skin irritations
rashes due to poison ivy, oak and sumac
dries the oozing and weeping of poison: ivy oak sumac
DO NOT USE on large areas of the body with any other product containing diphenhydramine, even one taken by mouth
Stop use and ask a doctor if
condition gets worse
symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
do not use more often than directed
adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
children under 2 years of age: ask a doctor
Inactive ingredients
cetyl alcohol, methylparaben, polysorbate 60, propylene glycol, purified water, sorbitan monostearate
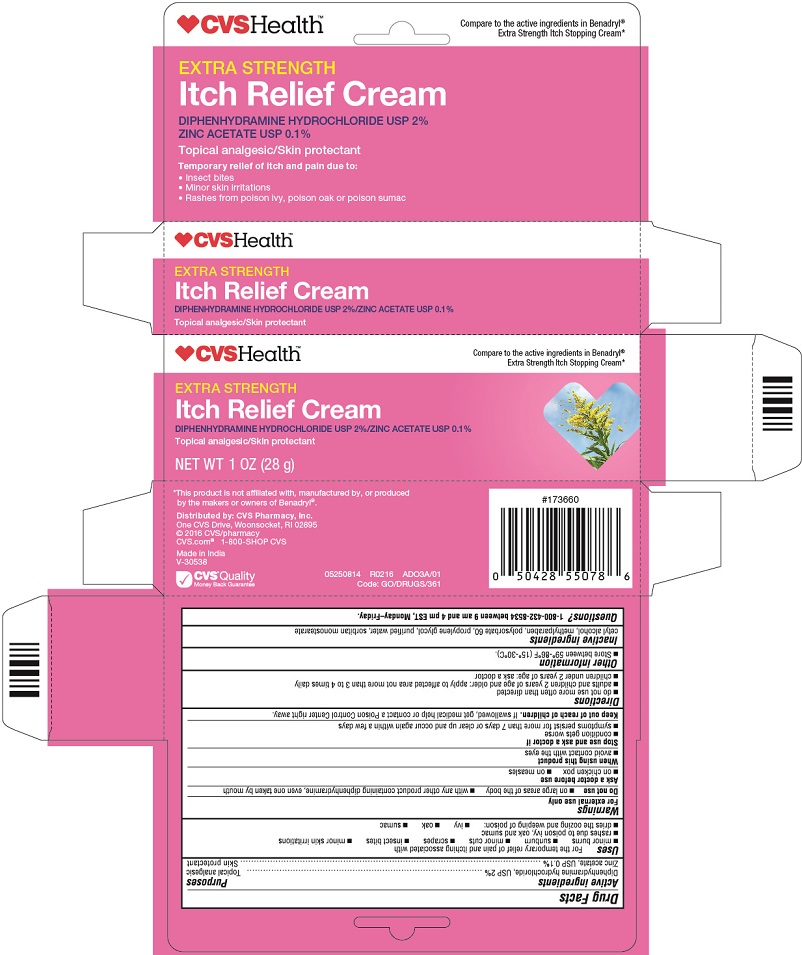
Principal Display Panel
CVS Health™
Compare to the active ingredients in Benadryl®
Extra Strength Itch Stopping Cream*
NDC: 59779-789-56
EXTRA STRENGTH
Itch Relief Cream
Diphenhydramine HYDROCHLORIDE USP 2%
Zinc Acetate USP 0.1%
Topical analgesic / Skin protectant
Temporary relief of itch and pain due to:
Insect bites
Minor skin irritations
Rashes from poison ivy, poison oak or poison sumac
NET WT 1 OZ (28g)
| ITCH RELIEF
EXTRA STRENGTH
diphenhydramine hydrochloride and zinc acetate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Teva Pharmaceuticals USA, Inc. (001627975) |