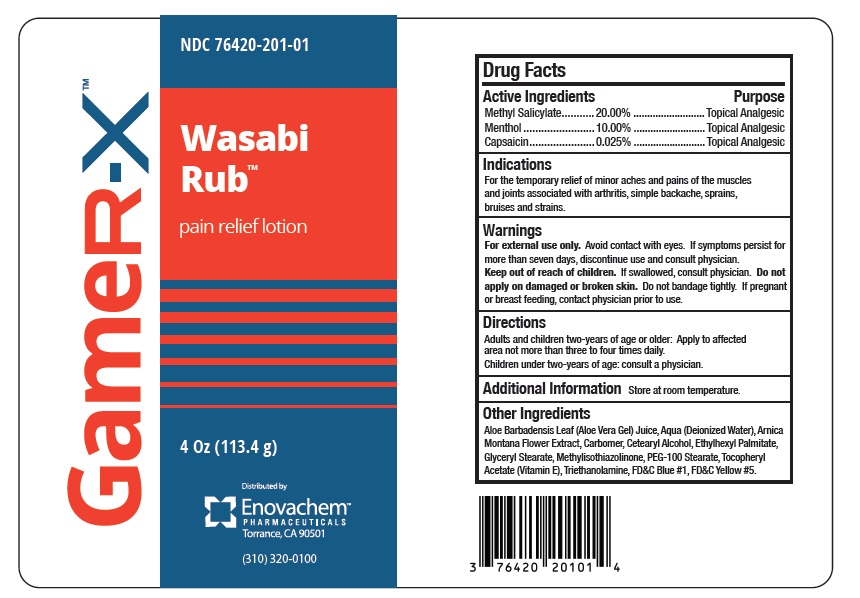
Wasabi Rub by Asclemed USA, Inc. Drug Facts
Wasabi Rub by
Drug Labeling and Warnings
Wasabi Rub by is a Otc medication manufactured, distributed, or labeled by Asclemed USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WASABI RUB- methyl salicylate, menthol, capsaicin lotion
Asclemed USA, Inc.
----------
Drug Facts
Indications
For the temporary relief of minor aches and pains of the muscles and joints associated with arthritis, simple backache, sprains, bruises and strains.
Directions
Adults and children two-years of age or older: Apply to affected area not more than three to four times daily.
Children under two-years of age: consult a physician.
Other Ingredients
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montana Flower Extract, Carbomer, Cetearyl Alcohol, Ethylexyl Palmitate, Glyceryl Stearate, Methylisothiazolinone, PEG-100 Stearate, Tocopheryl Acetate (Vitamin E), Triethanolamine, FD&C Blue#1 and FD&C Yellow#5.
| WASABI RUB
methyl salicylate, menthol, capsaicin lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Asclemed USA, Inc. (059888437) |
Trademark Results [Wasabi Rub]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WASABI RUB 78875771 3334814 Dead/Cancelled |
Robert P NIckell 2006-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.