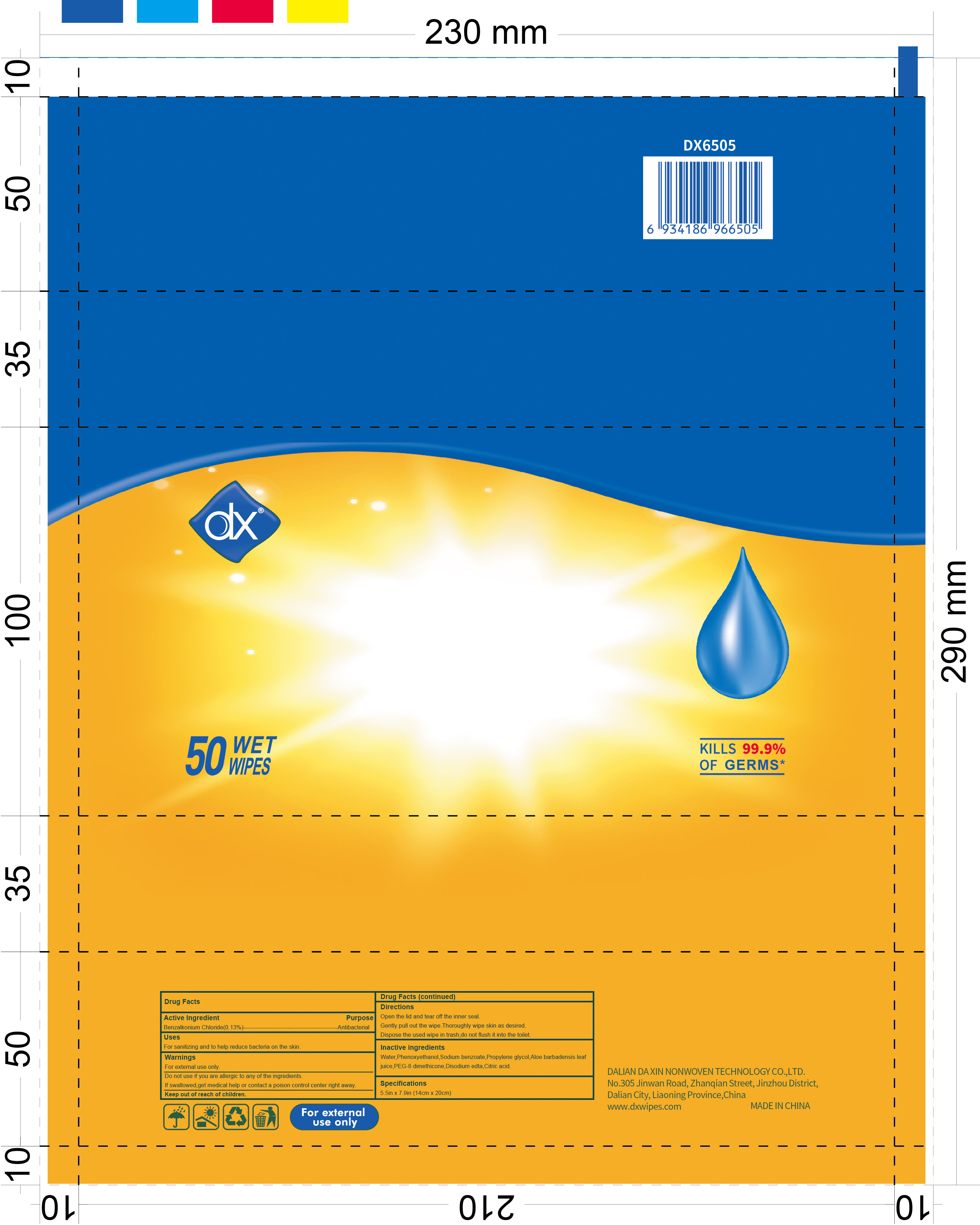
78028-005 WIPES 0.13% BENZALKONIUM CHLORIDE
WIPES by
Drug Labeling and Warnings
WIPES by is a Otc medication manufactured, distributed, or labeled by Dalian Daxin Nonwoven Technology Co., Ltd., Dalian Daxin Nonwoven Technology Co., Ltd. . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WIPES- benzalkonium chloride cloth
Dalian Daxin Nonwoven Technology Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
78028-005 WIPES 0.13% BENZALKONIUM CHLORIDE
Warning
For external use only.
Do not use if you are allergic to any of the ingredients.
If swallowed,get medical help or contact a poison control center right away.
Directions
Open the lid and tear off the inner seal.
Gently pull out the wipe.Thoroughly wipe skin as desired.
Dispose the used wipe in trash,do not flush it into the toilet.
| WIPES
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Dalian Daxin Nonwoven Technology Co., Ltd. (415363991) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dalian Daxin Nonwoven Technology Co., Ltd. | 415363991 | manufacture(78028-005) | |
Trademark Results [WIPES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WIPES 78748067 not registered Dead/Abandoned |
Drab, Francis, P. 2005-11-06 |
 WIPES 78581523 not registered Dead/Abandoned |
Drab, Francis P. 2005-03-07 |
 WIPES 77149616 3439269 Dead/Cancelled |
Nice-Pak Products, Inc. 2007-04-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.