Puricia Sanitizing Wipes (Blossom)
Puricia Sanitizing Wipes ( Blossom) by
Drug Labeling and Warnings
Puricia Sanitizing Wipes ( Blossom) by is a Otc medication manufactured, distributed, or labeled by PHARMABERG INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PURICIA SANITIZING WIPES ( BLOSSOM)- ethyl alcohol, benzalkonium chloride, cetylpyridinium chloride,didecyl dimethyl ammonium chloride, chlorphenesin, phenoxyethanol cloth
PHARMABERG INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
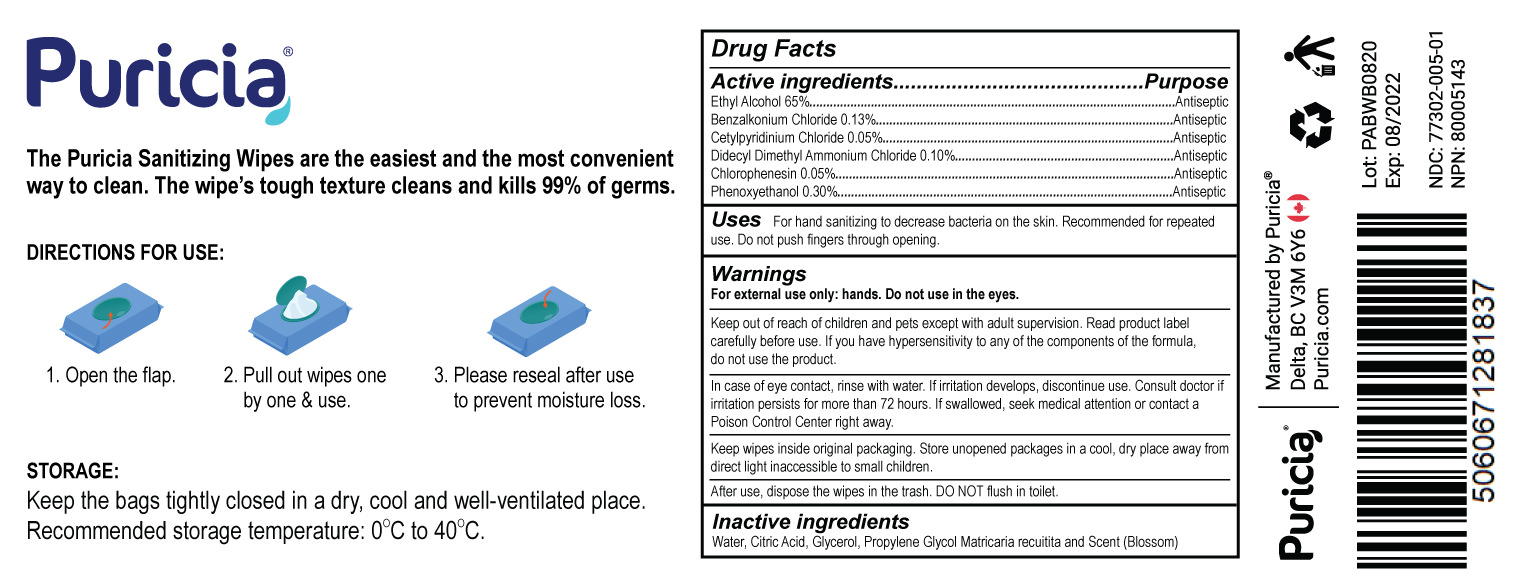
Puricia Sanitizing Wipes (Blossom)
The Puricia Sanitizing Wipes are the easiest and the most convenient way to clean. The wipe's tough texture cleans and kills 99% of germs.
DIRECTIONS FOR USE:
(Bag)
1. Open the flip.
2. Pull out wipes one by one & use.
3. Please reseal after use to prevent moisture loss.
(Canister)
1. Open the lid of the container. Tear open the foil
2. Thread first sheet in
CENTER of roll through slits in lid. Replace lid.
3. Pull out wipes one by one & use. Please reseal after use to prevent moisture loss.
STORAGE:
Keep the bags tightly closed in a dry, cool and well-ventilated place. Recommended storage temperature: 0°C to 40°C.
Active ingredients
Ethyl alcohol 65%
Benzalkonium Chloride 0.13%
Cetylpyridinium Chloride 0.05%
Didecyl Dimethyl Ammonium Chloride 0.10%
Chlorophenesin 0.05%
Phenoxyethanol 0.30%
Uses
For hand sanitizing to decrease bacteria on the skin. Recommended for repeated use. Do not push fingers through opening.
Read product label carefully before use.If you have a hypersensitivity to any of the components of the formula, do not use the product.
In case of eye contact ,rinse with water. If irritation develops, discontinue use. Consult doctor if irritation persists for more than 72 hours. If swallowed, seek medical attention or contact a Poison Control Center right away.
Keep wipes inside original packaging. Store unopened packages in a cool, dry place away from direct light inaccessible to small children.
After use, dispose the wipes in the trash. DO NOT flush in toilet.
| PURICIA SANITIZING WIPES ( BLOSSOM)
ethyl alcohol, benzalkonium chloride, cetylpyridinium chloride,didecyl dimethyl ammonium chloride, chlorphenesin, phenoxyethanol cloth |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - PHARMABERG INC. (204355565) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.