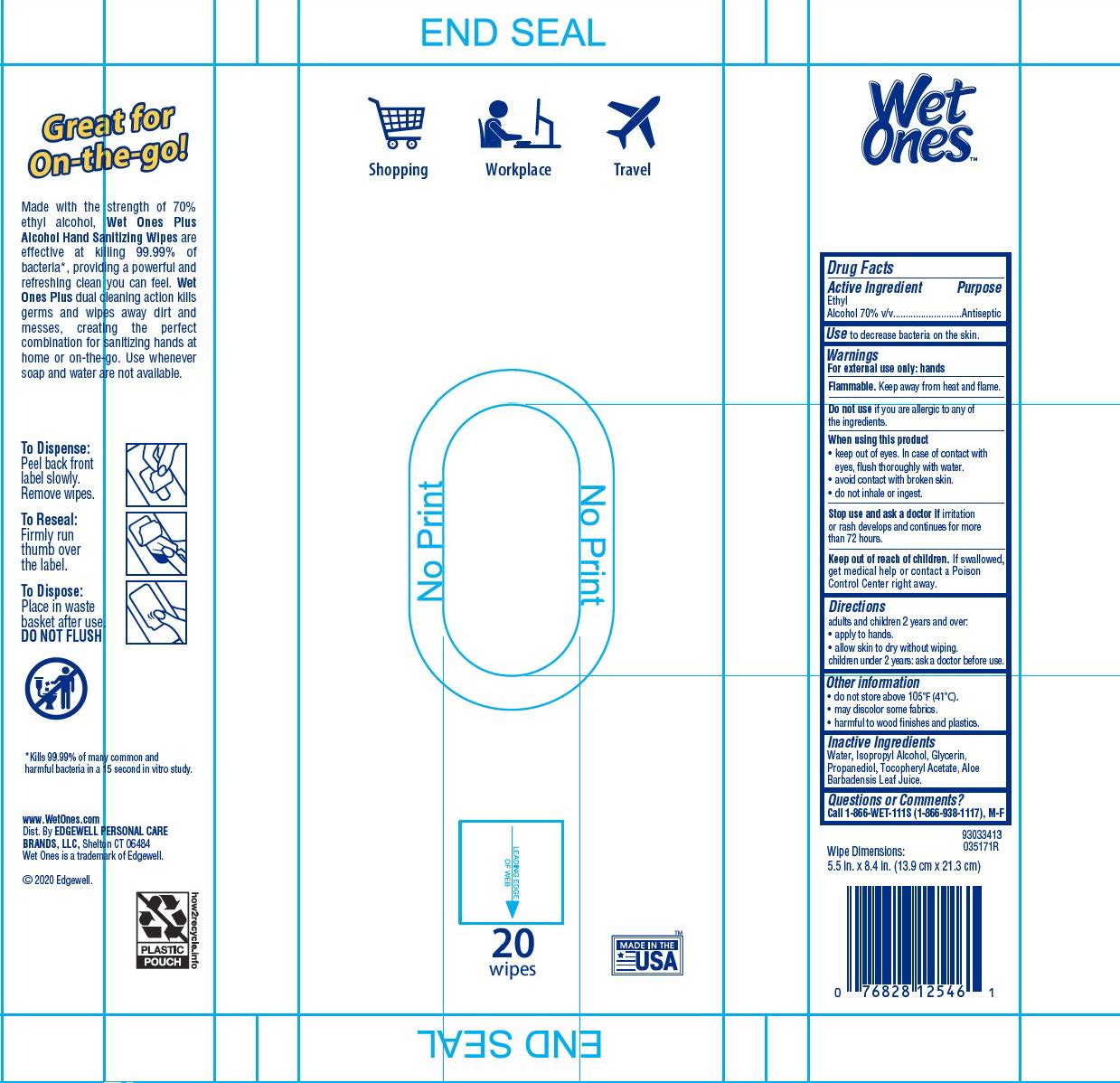
Wet Ones Hand Sanitizing Wipes Plus by Edgewell Personal Care Brands LLC
Wet Ones Hand Sanitizing Wipes Plus by
Drug Labeling and Warnings
Wet Ones Hand Sanitizing Wipes Plus by is a Otc medication manufactured, distributed, or labeled by Edgewell Personal Care Brands LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WET ONES HAND SANITIZING WIPES PLUS- ethyl alcohol swab
Edgewell Personal Care Brands LLC
----------
Warnings
For externak use only: hands
Flammable. Keep away from heat and flame
Directions
adults and children 2 years and over:
- apply to hands.
- allow skin to dry without wiping.
children under 2 years: ask a doctor before use
Other information
- do not store above 105℉ (41℃).
- may discolor some fabrics
- harmful to wood finishes and plastics
| WET ONES HAND SANITIZING WIPES PLUS
ethyl alcohol swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Edgewell Personal Care Brands LLC (151179769) |
Revised: 10/2024
Document Id: 2371bff7-9ac3-6ede-e063-6394a90a6c6d
Set id: b338ddab-0908-f539-e053-2995a90a3c1a
Version: 2
Effective Time: 20241001