Asutra WellHeal by BioLyte Laboratories, LLC
Asutra WellHeal by
Drug Labeling and Warnings
Asutra WellHeal by is a Homeopathic medication manufactured, distributed, or labeled by BioLyte Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ASUTRA WELLHEAL- salix alba bark, stellaria media, comfrey root, horse chestnut, daphne mezereum bark, equisetum arvense top, urtica urens, althaea officinalis root, hamamelis virginiana root bark/stem bark, calendula officinalis flowering top cream
BioLyte Laboratories, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
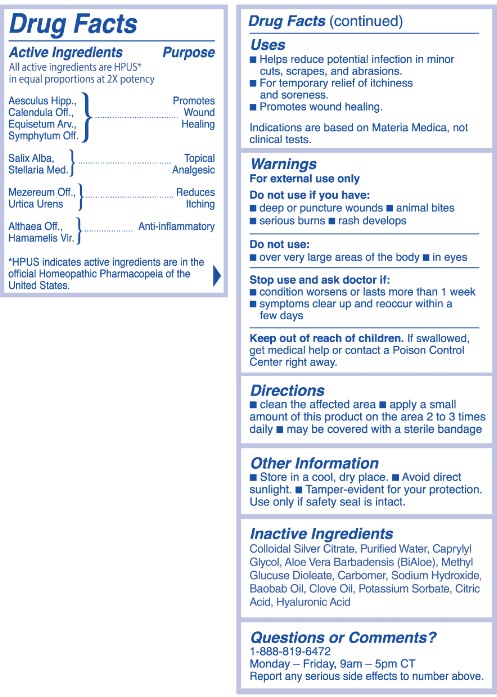
Active Ingredients
All active ingredients are HPUS* in equal proportions at 2X potency
Aesculus Hipp.,
Caledndula Off.,
Equisetum Arv.,
Symphytum Off.
Salix Alba,
Stellaria Med.
Althaea Off.,
Hamamelis Vir.
*HPUS indicates active ingredients are in the official Homeopathic Pharmacopeia of the United States.
Active Ingredient Purpose
Aesculus Hipp.,
Caledndula Off.,
Equisetum Arv.,
Symphytum Off...................................... Promotes Wound Healing
Salix Alba,
Stellaria Med..........................................Topical Analgesic
Althaea Off.,
Hamamelis Vir........................................Anti-inflammatory
Uses
Helps reduce potential infection in minor cuts, scrapes, and abraisons.
For temporary relief of itchiness.
Promotes wound healing and soreness.
Indications are based on Materia Medica, not clinical tests.
Do not use if you have:
- deep or puncture wounds
- animal bites
- serious burns
- rash develops
Do not use:
- over very large areas of the body
- in the eyes
Stop use and ask doctor if:
- condition worsens or lasts more than 1 week
- symptopms clear up and reoccur within a few days.
Stop use and ask doctor if:
- condition worsens or lasts more than 1 week
- symptopms clear up and reoccur within a few days.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area
- apply a small amount of this product on the area 2 to 3 times daily
- may be covered with a sterile bandage
Other Information
- Store in a cool, dry place
- Avoid direct sunlight.
- Tamper-evident for your protection. Use only if safety seal is intact.
Inactive Ingredients:
Colloidal Silver Citrate, Purified Water, Caprylyl Glycol, Aloe Vera Barbadensis (BiAloe), Methyl Glucuse Diolate, Carbomer, Sodium Hydroxide, Baobab Oil, Clove Oil, Potassium Sorbate, Citric Acid, Hyaluronic Acid
| ASUTRA WELLHEAL
salix alba bark, stellaria media, comfrey root, horse chestnut, daphne mezereum bark, equisetum arvense top, urtica urens, althaea officinalis root, hamamelis virginiana root bark/stem bark, calendula officinalis flowering top cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - BioLyte Laboratories, LLC (015560564) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioLyte Laboratories, LLC | 015560564 | manufacture(58368-010) | |