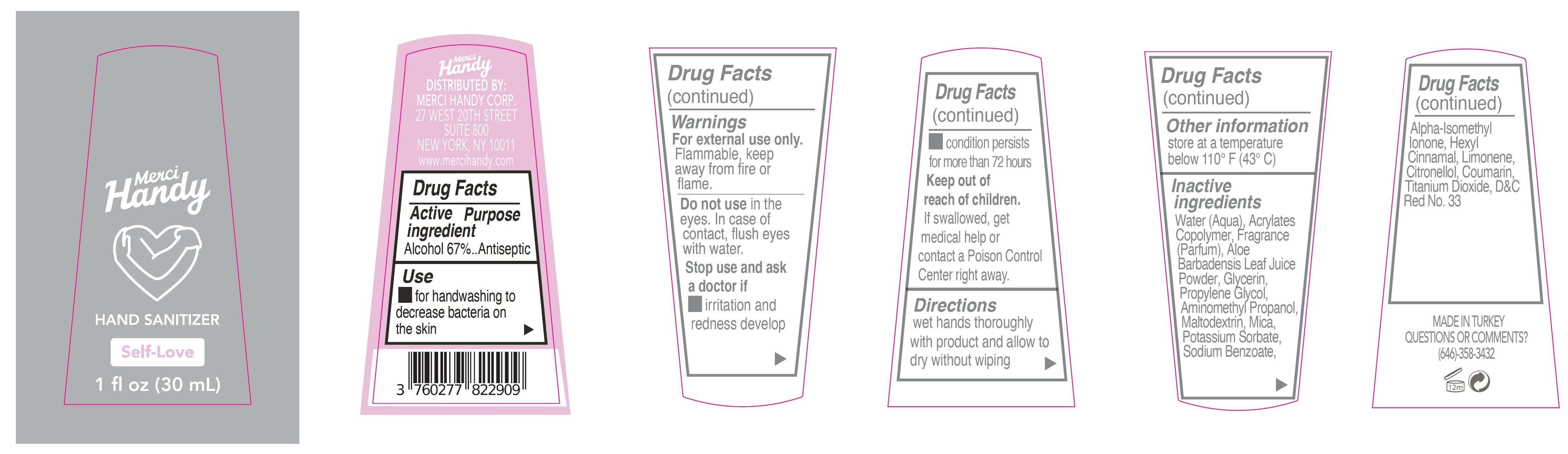
Merci Handy Hand Sanitizer Self-Love
Merci Handy Hand Sanitizer Self-Love by
Drug Labeling and Warnings
Merci Handy Hand Sanitizer Self-Love by is a Otc medication manufactured, distributed, or labeled by MERCI HANDY CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MERCI HANDY HAND SANITIZER SELF-LOVE- alcohol gel
MERCI HANDY CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Merci Handy Hand Sanitizer Self-Love
Warnings
For external use only.
Flammable, keep away from fire or flame.
Inactive ingredients
Water (Aqua), Acrylates Copolymer, Fragrance (Parfum), Aloe Barbadensis Leaf Juice Powder, Glycerin, Propylene Glycol, Aminomethyl propanol, Maltodextrin, Mica, Potassium Sorbate, Sodium Benzoate,Alpha-Isomethyl Ionone, Hexyl Cinnamal, Limonene, Citronellol, Coumarin, Titanium Dioxide, D&C Red No. 33
| MERCI HANDY HAND SANITIZER SELF-LOVE
alcohol gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - MERCI HANDY CORPORATION (118006306) |