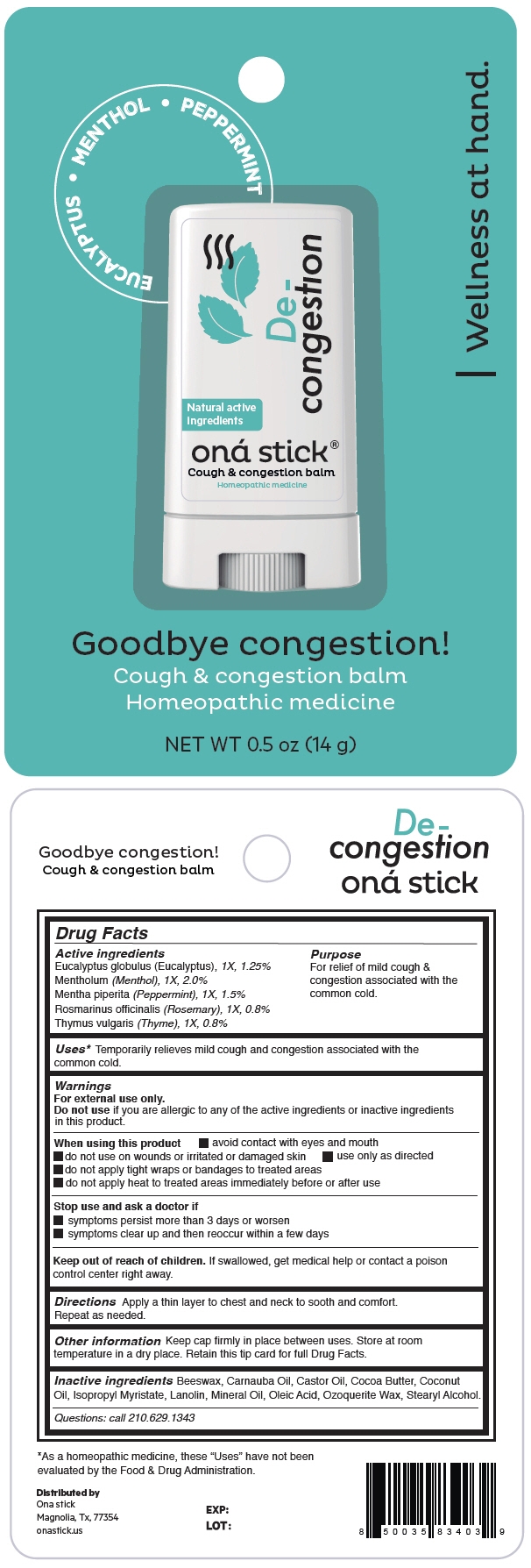
DE-CONGESTION- eucalyptus globulus leaf, menthol, unspecified form, peppermint, rosemary and thymus vulgaris leaf stick
De-congestion by
Drug Labeling and Warnings
De-congestion by is a Homeopathic medication manufactured, distributed, or labeled by ONA STICK USA LLC, Abinter Labs LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses1
Temporarily relieves mild cough and congestion associated with the common cold.
- 1 As a homeopathic medicine, these "Uses" have not been evaluated by the Food & Drug Administration.
-
Warnings
For external use only.
Do not use if you are allergic to any of the active ingredients or inactive ingredients in this product.
When using this product
- avoid contact with eyes and mouth
- do not use on wounds or irritated or damaged skin
- use only as directed
- do not apply tight wraps or bandages to treated areas
- do not apply heat to treated areas immediately before or after use
- Directions
- Other information
- Inactive ingredients
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14 g Tube Blister Pack
-
INGREDIENTS AND APPEARANCE
DE-CONGESTION
eucalyptus globulus leaf, menthol, unspecified form, peppermint, rosemary and thymus vulgaris leaf stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82610-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 1 [hp_X] in 14 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 [hp_X] in 14 g PEPPERMINT (UNII: V95R5KMY2B) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT 1 [hp_X] in 14 g ROSEMARY (UNII: IJ67X351P9) (ROSEMARY - UNII:IJ67X351P9) ROSEMARY 1 [hp_X] in 14 g THYMUS VULGARIS LEAF (UNII: GRX3499643) (THYMUS VULGARIS LEAF - UNII:GRX3499643) THYMUS VULGARIS LEAF 1 [hp_X] in 14 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CARNAUBA WAX (UNII: R12CBM0EIZ) Castor Oil (UNII: D5340Y2I9G) Cocoa Butter (UNII: 512OYT1CRR) Coconut Oil (UNII: Q9L0O73W7L) Isopropyl Myristate (UNII: 0RE8K4LNJS) Lanolin (UNII: 7EV65EAW6H) Mineral Oil (UNII: T5L8T28FGP) Oleic Acid (UNII: 2UMI9U37CP) CERESIN (UNII: Q1LS2UJO3A) Stearyl Alcohol (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82610-004-14 1 in 1 BLISTER PACK 03/15/2022 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 03/15/2022 Labeler - ONA STICK USA LLC (118525613) Establishment Name Address ID/FEI Business Operations Abinter Labs LLC 117981053 MANUFACTURE(82610-004)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.