DIDANOSINE capsule, delayed release
Didanosine by
Drug Labeling and Warnings
Didanosine by is a Prescription medication manufactured, distributed, or labeled by Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DIDANOSINE DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for DIDANOSINE DELAYED-RELEASE CAPSULES.
DIDANOSINE delayed-release capsules, for oral use
(Enteric-Coated Beadlets)
Initial U.S. Approval: 1991WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
See full prescribing information for complete boxed warning.
- Fatal and nonfatal pancreatitis. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis. (5.1)
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine. (5.2)
Coadministration of didanosine delayed-release capsules with stavudine is contraindicated. (4)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Didanosine delayed-release capsules are a nucleoside reverse transcriptase inhibitor for use in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV)-1 infection. (1)
DOSAGE AND ADMINISTRATION
- Adult patients: Administered on an empty stomach. Dosing is based on body weight. (2.1)
- Pediatric patients: Ages 6 to 18 years, can safely swallow capsules and body weight at least 20 kg. Administered on an empty stomach, dosing is based on body weight. (2.1)
Body Weight
Dose
20 kg to less than 25 kg
200 mg once daily
25 kg to less than 60 kg
250 mg once daily
at least 60 kg
400 mg once daily
DOSAGE FORMS AND STRENGTHS
Capsules: 125 mg, 200 mg, 250 mg, 400 mg (3)
CONTRAINDICATIONS
Coadministration with stavudine, allopurinol, or ribavirin is contraindicated. (4)
WARNINGS AND PRECAUTIONS
- Pancreatitis: Suspension or discontinuation of didanosine may be necessary. (5.1) Coadministration of didanosine delayed-release capsules with stavudine is contraindicated. (4)
- Lactic acidosis and severe hepatomegaly with steatosis: Suspend didanosine in patients who develop clinical symptoms or signs with or without laboratory findings. (5.2)
- Hepatic toxicity: Interruption or discontinuation of didanosine must be considered upon worsening of liver disease. (5.3) Coadministration of didanosine delayed-release capsules with stavudine is contraindicated. (4)
- Non-cirrhotic portal hypertension: Discontinue didanosine in patients with evidence of non-cirrhotic portal hypertension. (5.4)
- Patients may develop peripheral neuropathy (5.5), retinal changes and optic neuritis (5.6), immune reconstitution syndrome (5.7), and lipoatrophy (5.8).
ADVERSE REACTIONS
- In adults, the most common adverse reactions (greater than 10%, all grades) are diarrhea, peripheral neurologic symptoms/neuropathy, nausea, headache, rash, and vomiting. (6.1)
- Adverse reactions in pediatric patients were consistent with those in adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: Fatal lactic acidosis has been reported in pregnant individuals who received both didanosine and stavudine with other agents. Coadministration of didanosine delayed-release capsules with stavudine is contraindicated. (4, 5.2, 8.1)
- Lactation: Breastfeeding is not recommended due to the potential for HIV-1 transmission. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage (Adult and Pediatric Patients)
2.2 Renal Impairment
2.3 Dose Adjustment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
5.2 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.3 Hepatic Toxicity
5.4 Non-cirrhotic Portal Hypertension
5.5 Peripheral Neuropathy
5.6 Retinal Changes and Optic Neuritis
5.7 Immune Reconstitution Syndrome
5.8 Lipoatrophy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Established Drug Interactions
7.2 Predicted Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis [see Warnings and Precautions (5.1)].
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine with other antiretroviral agents. Coadministration of didanosine delayed-release capsules and stavudine is contraindicated because of increased risk of serious and/or life-threatening events [see Contraindications (4) and Warnings and Precautions (5.2)]. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occurs.
-
1 INDICATIONS AND USAGE
Didanosine delayed-release capsules, also known as ddI, in combination with other antiretroviral agents are indicated for the treatment of human immunodeficiency virus (HIV)-1 infection [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
Didanosine delayed-release capsules should be administered on an empty stomach. Didanosine delayed-release capsules should be swallowed intact.
2.1 Recommended Dosage (Adult and Pediatric Patients)
The recommended total daily dose is based on body weight and is administered as one capsule given on a once-daily schedule as outlined in Table 1.
The recommended total daily dose to be administered once daily to pediatric patients weighing at least 20 kg who can swallow capsules is based on body weight (kg), consistent with the recommended adult dosing guidelines (see Table 1). Please consult the complete prescribing information for didanosine pediatric powder for oral solution for dosage and administration of didanosine to pediatric patients weighing less than 20 kg or who can not swallow capsules.
Table 1: Recommended Dosage (Adult and Pediatric Patients)
Body Weight
Dose
20 kg to less than 25 kg
200 mg once daily
25 kg to less than 60 kg
250 mg once daily
at least 60 kg
400 mg once daily
2.2 Renal Impairment
Dosing recommendations for didanosine delayed-release capsules and didanosine pediatric powder for oral solution are different for patients with renal impairment. Please consult the complete prescribing information on administration of didanosine pediatric powder for oral solution to patients with renal impairment.
Adult Patients
In adult patients with impaired renal function, the dose of didanosine delayed-release capsules should be adjusted to compensate for the slower rate of elimination. The recommended doses and dosing intervals of didanosine delayed-release capsules in adult patients with renal insufficiency are presented in Table 2.
Table 2: Recommended Dosage in Patients with Renal Impairment by Body Weighta Creatinine
Clearance
(mL/min)Dosage (mg) at least 60 kg less than 60 kg a Based on studies using a buffered formulation of didanosine.
b Not suitable for use in patients less than 60 kg with CLcr less than 10 mL/min. An alternate formulation of didanosine should be used.
at least 60
30 to 59
10 to 29
less than 10
400 once daily
200 once daily
125 once daily
125 once daily
250 once daily
125 once daily
125 once daily
b
Pediatric Patients
Urinary excretion is also a major route of elimination of didanosine in pediatric patients, therefore the clearance of didanosine may be altered in pediatric patients with renal impairment. Although there are insufficient data to recommend a specific dose adjustment of didanosine delayed-release capsules in this patient population, a reduction in the dose should be considered (see Table 2).
Patients Requiring Continuous Ambulatory Peritoneal Dialysis (CAPD) or Hemodialysis
For patients requiring CAPD or hemodialysis, follow dosing recommendations for patients with creatinine clearance of less than 10 mL/min, shown in Table 2. It is not necessary to administer a supplemental dose of didanosine following hemodialysis.2.3 Dose Adjustment
Concomitant Therapy with Tenofovir Disoproxil Fumarate
In patients who are also taking tenofovir disoproxil fumarate, a dose reduction of didanosine delayed-release capsules to 250 mg (adults weighing at least 60 kg with creatinine clearance of at least 60 mL/min) or 200 mg (adults weighing less than 60 kg with creatinine clearance of at least 60 mL/min) once daily taken together with tenofovir disoproxil fumarate and a light meal (400 kilocalories or less, 20% fat or less) or in the fasted state is recommended. The appropriate dose of didanosine delayed-release capsules coadministered with tenofovir disoproxil fumarate in patients with creatinine clearance of less than 60 mL/min has not been established [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
Hepatic Impairment
No dose adjustment is required in patients with hepatic impairment [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]. -
3 DOSAGE FORMS AND STRENGTHS
Didanosine delayed-release capsules USP:
- 125 mg are white / white size ‘3’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘70’ on white body with black edible ink filled with white to off-white beadlets.
- 200 mg are white / white size ‘1’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘69’ on white body with black edible ink filled with white to off-white beadlets.
- 250 mg are white / white size ‘0’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘10’ on white body with black edible ink filled with white to off-white beadlets.
- 400 mg are white / white size ‘00’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘09’ on white body with black edible ink filled with white to off-white beadlets.
-
4 CONTRAINDICATIONS
Didanosine delayed-release capsules are contraindicated when coadministered with the following medications:
- Stavudine- potential for serious and/or life-threatening events, notably pancreatitis, lactic acidosis, hepatotoxicity, and peripheral neuropathy [see Warnings and Precautions (5.1, 5.2, 5.3, 5.5)].
- Allopurinol- systemic exposures of didanosine are increased, which may increase didanosine-associated toxicity [see Clinical Pharmacology (12.3)].
- Ribavirin- exposures of the active metabolite of didanosine (dideoxyadenosine 5′-triphosphate) are increased. Fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in patients receiving both didanosine and ribavirin.
-
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with signs or symptoms of pancreatitis and discontinued in patients with confirmed pancreatitis. Patients treated with didanosine delayed-release capsules in combination with stavudine may be at increased risk for pancreatitis; the coadministration of didanosine delayed-release capsules and stavudine is contraindicated [see Contraindications (4)].
When treatment with life-sustaining drugs known to cause pancreatic toxicity is required, suspension of didanosine delayed-release capsules therapy is recommended. In patients with risk factors for pancreatitis, didanosine delayed-release capsules should be used with extreme caution and only if clearly indicated. Patients with advanced HIV-1 infection, especially the elderly, are at increased risk of pancreatitis and should be followed closely. Patients with renal impairment may be at greater risk for pancreatitis if treated without dose adjustment. The frequency of pancreatitis is dose related [see Adverse Reactions (6)].5.2 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine with other antiretroviral agents. Coadministration of didanosine delayed-release capsules and stavudine is contraindicated [see Contraindications (4) and Use in Specific Populations (8.1)]. Particular caution should be exercised when administering didanosine delayed-release capsules to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with didanosine delayed-release capsules should be suspended in any patient who develops clinical signs or symptoms with or without laboratory findings consistent with symptomatic hyperlactatemia, lactic acidosis, or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.3 Hepatic Toxicity
The safety and efficacy of didanosine delayed-release capsules have not been established in HIV-infected patients with significant underlying liver disease. During combination antiretroviral therapy, patients with preexisting liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities, including severe and potentially fatal hepatic adverse events, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Hepatotoxicity and hepatic failure resulting in death were reported during postmarketing surveillance in HIV-infected patients treated with hydroxyurea and other antiretroviral agents. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. Coadministration of didanosine delayed-release capsules and stavudine is contraindicated; the combination of didanosine delayed-release capsules and hydroxyurea should be avoided [see Contraindications (4) and Drug Interactions (7.2)].5.4 Non-cirrhotic Portal Hypertension
Postmarketing cases of non-cirrhotic portal hypertension have been reported, including cases leading to liver transplantation or death. Cases of didanosine-associated non-cirrhotic portal hypertension were confirmed by liver biopsy in patients with no evidence of viral hepatitis. Onset of signs and symptoms ranged from months to years after start of didanosine therapy. Common presenting features included elevated liver enzymes, esophageal varices, hematemesis, ascites, and splenomegaly.
Patients receiving didanosine delayed-release capsules should be monitored for early signs of portal hypertension (e.g., thrombocytopenia and splenomegaly) during routine medical visits. Appropriate laboratory testing including liver enzymes, serum bilirubin, albumin, complete blood count, and international normalized ratio (INR) and ultrasonography should be considered. Didanosine delayed-release capsules should be discontinued in patients with evidence of non-cirrhotic portal hypertension.5.5 Peripheral Neuropathy
Peripheral neuropathy, manifested by numbness, tingling, or pain in the hands or feet, has been reported in patients receiving didanosine therapy. Peripheral neuropathy has occurred more frequently in patients with advanced HIV disease, in patients with a history of neuropathy, or in patients being treated with neurotoxic drug therapy. Discontinuation of didanosine delayed-release capsules should be considered in patients who develop peripheral neuropathy [see Contraindications (4), Adverse Reactions (6), and Drug Interactions (7.2)].
5.6 Retinal Changes and Optic Neuritis
Retinal changes and optic neuritis have been reported in patients taking didanosine. Periodic retinal examinations should be considered for patients receiving didanosine delayed-release capsules [see Adverse Reactions (6)].
5.7 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including didanosine delayed-release capsules. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.5.8 Lipoatrophy
Treatment with didanosine delayed-release capsules has been associated with loss of subcutaneous fat, which is most evident in the face, limbs, and buttocks. The incidence and severity of lipoatrophy are related to cumulative exposure, and is often not reversible when didanosine delayed-release capsules treatment is stopped. Patients receiving didanosine delayed-release capsules should be frequently examined and questioned for signs of lipoatrophy, and if feasible, therapy should be switched to an alternative regimen if there is suspicion of lipoatrophy.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections:
- Pancreatitis [see Warnings and Precautions (5.1)]
- Lactic acidosis/severe hepatomegaly with steatosis [see Warnings and Precautions (5.2)]
- Hepatic toxicity [see Warnings and Precautions (5.3)]
- Non-cirrhotic portal hypertension [see Warnings and Precautions (5.4)]
- Peripheral neuropathy [see Warnings and Precautions (5.5)]
- Retinal changes and optic neuritis [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Subjects
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients. Selected clinical adverse reactions that occurred in combination with other antiretroviral agents are provided in Table 3.
Table 3: Selected Clinical Adverse Reactions, Study AI454-152a Adverse Reactions Percent of Patientsb,c didanosine delayed-release
capsules + stavudine +
nelfinavir
n=258zidovudine/lamivudined +
nelfinavir
n=253a Median duration of treatment was 62 weeks in the didanosine delayed-release capsules + stavudine + nelfinavir group and 61 weeks in the zidovudine/lamivudine + nelfinavir group.
b Percentages based on treated patients.
c The incidences reported included all severity grades and all reactions regardless of causality.
d Zidovudine/lamivudine combination tablet.
* This event was not observed in this study arm.
Diarrhea
Peripheral Neurologic Symptoms/Neuropathy
Nausea
Headache
Rash
Vomiting
Pancreatitis (see below)
57
25
24
22
14
14
less than 1
58
11
36
17
12
19
*
In clinical trials using a buffered formulation of didanosine, pancreatitis resulting in death was observed in one patient who received didanosine plus stavudine plus nelfinavir, one patient who received didanosine plus stavudine plus indinavir, and 2 of 68 patients who received didanosine plus stavudine plus indinavir plus hydroxyurea. In an early access program, pancreatitis resulting in death was observed in one patient who received didanosine delayed-release capsules plus stavudine plus hydroxyurea plus ritonavir plus indinavir plus efavirenz [see Warnings and Precautions (5)].
The frequency of pancreatitis is dose related. In phase 3 studies with buffered formulations of didanosine, incidence ranged from 1% to 10% with doses higher than are currently recommended and 1% to 7% with recommended dose.
Selected laboratory abnormalities that occurred in a study of didanosine delayed-release capsules in combination with other antiretroviral agents are shown in Table 4.
Table 4: Selected Laboratory Abnormalities, Study AI454-152a Percent of Patientsb didanosine delayed-release capsules + stavudine
+ nelfinavir
n=258zidovudine/lamivudinec
+ nelfinavir
n=253Parameter Grades 3 to 4d All Grades Grades 3 to 4d All Grades a Median duration of treatment was 62 weeks in the didanosine delayed-release capsules + stavudine + nelfinavir group and 61 weeks in the zidovudine/lamivudine + nelfinavir group.
b Percentages based on treated patients.
c Zidovudine/lamivudine combination tablet.
d Greater than 5 x ULN for SGOT and SGPT, at least 2.1 x ULN for lipase, and at least 2.6 x ULN for bilirubin (ULN = upper limit of normal).
SGOT (AST)
SGPT (ALT)
Lipase
Bilirubin
5
6
5
less than 1
46
44
23
9
5
5
2
less than 1
19
22
13
3
Clinical Trials Experience in Pediatric Patients
In clinical trials, 743 pediatric patients between 2 weeks and 18 years of age have been treated with didanosine. Adverse reactions and laboratory abnormalities reported to occur in these patients were generally consistent with the safety profile of didanosine in adults.
In pediatric phase 1 studies, pancreatitis occurred in 2 of 60 (3%) patients treated at entry doses below 300 mg/m2/day and in 5 of 38 (13%) patients treated at higher doses. In study ACTG 152, pancreatitis occurred in none of the 281 pediatric patients who received didanosine 120 mg/m2 every 12 hours and in less than 1% of the 274 pediatric patients who received didanosine 90 mg/m2 every 12 hours in combination with zidovudine [see Clinical Studies (14)].
Retinal changes and optic neuritis have been reported in pediatric patients.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine delayed-release capsules or a combination of these factors.
Blood and Lymphatic System Disorders - anemia, leukopenia, and thrombocytopenia.
Body as a Whole - abdominal pain, alopecia, anaphylactoid reaction, asthenia, chills/fever, pain.
Digestive Disorders - anorexia, dyspepsia, and flatulence.
Exocrine Gland Disorders - pancreatitis (including fatal cases) [see Warnings and Precautions (5.1)], sialoadenitis, parotid gland enlargement, dry mouth, and dry eyes.
Hepatobiliary Disorders - symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis [see Warnings and Precautions (5.2)]; non-cirrhotic portal hypertension [see Warnings and Precautions (5.4)]; hepatitis and liver failure.
Metabolic Disorders - diabetes mellitus, elevated serum alkaline phosphatase level, elevated serum amylase level, elevated serum gamma-glutamyltransferase level, elevated serum uric acid level, hypoglycemia, and hyperglycemia.
Musculoskeletal Disorders - myalgia (with or without increases in creatine kinase), rhabdomyolysis including acute renal failure and hemodialysis, arthralgia, and myopathy.
Ophthalmologic Disorders - retinal depigmentation and optic neuritis [see Warnings and Precautions (5.6)]. -
7 DRUG INTERACTIONS
7.1 Established Drug Interactions
Clinical recommendations based on the results of drug interaction studies are listed in Table 5. Pharmacokinetic results of drug interaction studies are shown in Tables 9 to 12 [see Contraindications (4), Clinical Pharmacology (12.3)].
Table 5: Established Drug Interactions Based on Studies with Didanosine Delayed-Release Capsules or Studies with Buffered Formulations of Didanosine and Expected to Occur with Didanosine Delayed-Release Capsules Drug Effect Clinical Comment ↑ Indicates increase.
↓ Indicates decrease.
a Coadministration of didanosine with food decreases didanosine concentrations. Thus, although not studied, it is possible that coadministration with heavier meals could reduce didanosine concentrations further.
ganciclovir
↑ didanosine concentration
If there is no suitable alternative to ganciclovir, then use in combination with didanosine delayed-release capsules with caution. Monitor for didanosine-
associated toxicity.
methadone
↓ didanosine concentration
If coadministration of methadone and didanosine is necessary, the recommended formulation of didanosine is didanosine delayed-release capsules. Patients
should be closely monitored for adequate clinical response when didanosine delayed-release capsules are coadministered with methadone, including monitoring
for changes in HIV RNA viral load. Do not coadminister methadone with didanosine pediatric powder due to significant decreases in didanosine concentrations.
nelfinavir
No interaction 1 hour after didanosine
Administer nelfinavir 1 hour after didanosine delayed-release capsules.
tenofovir disoproxil fumarate
↑ didanosine concentration
A dose reduction of didanosine delayed-release capsules to the following dosage once daily taken together with tenofovir disoproxil fumarate and a light meal
(400 kilocalories or less and 20% fat or less) or in the fasted state is recommended.a
- 250 mg (adults weighing at least 60 kg with creatinine clearance of at least 60 mL/min)
- 200 mg (adults weighing less than 60 kg with creatinine clearance of at least 60 mL/min)
Exposure to didanosine is increased when coadministered with tenofovir disoproxil fumarate [Table 5 and see Clinical Pharmacokinetics (12.3, Tables 9 and 10)]. Increased exposure may cause or worsen didanosine-related clinical toxicities, including pancreatitis, symptomatic hyperlactatemia/lactic acidosis, and peripheral neuropathy. Coadministration of tenofovir disoproxil fumarate with didanosine delayed-release capsules should be undertaken with caution, and patients should be monitored closely for didanosine-related toxicities and clinical response. Didanosine delayed-release capsules should be suspended if signs or symptoms of pancreatitis, symptomatic hyperlactatemia, or lactic acidosis develop [see Dosage and Administration (2.3), Warnings and Precautions (5)]. Suppression of CD4 cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine at a dose of 400 mg daily.
7.2 Predicted Drug Interactions
Predicted drug interactions with didanosine delayed-release capsules are listed in Table 6.
Table 6: Predicted Drug Interactions with Didanosine Delayed-Release Capsules ↑ Indicates increase.
a Only if other drugs are not available and if clearly indicated. If treatment with life-sustaining drugs that cause pancreatic toxicity is required, suspension of didanosine delayed-release capsules is recommended [see Warnings and Precautions (5.1)].
b [see Warnings and Precautions (5.6)].
Drug or Drug Class
Effect
Clinical Comment
Drugs that may cause pancreatic toxicity
↑ risk of pancreatitis
Use only with extreme caution.a
Neurotoxic drugs
↑ risk of neuropathy
Use with caution.b
Hydroxyurea
↑ risk of pancreatitis, fatal hepatotoxicity, and severe peripheral neuropathy
Use should be avoided.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to didanosine delayed-release capsules during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine with other antiretroviral agents. It is unclear if pregnancy augments the risk of lactic acidosis/hepatic steatosis syndrome reported in nonpregnant individuals receiving nucleoside analogues [see Warnings and Precautions (5.2)]. Coadministration of didanosine delayed-release capsules and stavudine is contraindicated [see Contraindications (4)].
Based on APR reports, congenital malformations were reported when administered during pregnancy. The prevalence of birth defects was 4.7% in the first trimester compared with 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) and 4.2% in the Texas Birth Defects Registry (TBDR) (see Data). No pattern of defects was identified by the APR. Based on these findings, the clinical relevance is uncertain.
The rate of miscarriage is not reported in the APR. In the U.S. general population, the estimated background risks of miscarriage in clinically recognized pregnancies is 15 to 20%, respectively.
In animal reproduction studies, no evidence of adverse developmental outcomes was observed with didanosine at systemic exposures (AUC) up to 12 (rats) and 14 (rabbits) times the exposure in humans at the recommended daily human dose of didanosine delayed-release capsules (see Data).
Clinical Considerations
Maternal Adverse Reactions
Cases of lactic acidosis syndrome, sometimes fatal, have occurred in pregnant individuals using didanosine delayed-release capsules in combination with stavudine. Didanosine delayed-release capsules is associated with an increased risk of lactic acidosis syndrome/hepatic steatosis syndrome [see Warnings and Precautions (5.2)]
Data
Human Data
Based on prospective reports to the APR of exposure to didanosine-containing regimens during pregnancy (including 427 exposed in the first trimester and 462 exposed in the second/third trimester), the prevalence of birth defects in live births was 4.7% (95% CI: 2.9% to 7.1%) with first trimester exposure to didanosine-containing regimens and 4.3% (95% CI: 2.7% to 6.6%) with the second/third trimester exposure to didanosine-containing regimens compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP and 4.2% in the TBDR.
Prospective reports from the APR of overall major birth defects in pregnancies exposed to didanosine delayed-release capsules is compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP and TBDR as the external comparator groups. Limitations of using external comparators include differences in methodology and populations, as well as confounding due to the underlying disease.
Animal Data
Didanosine was administered orally at up to 1000 mg per kg daily to pregnant rats and at up to 600 mg per kg daily to pregnant rabbits on gestation Days 7 to 17 and 6 to 18, respectively, and also to rats 14 days before mating through weaning. No adverse effects on embryo-fetal development (rats and rabbits) were observed up to the highest dose tested. During organogenesis, systemic exposures (AUC) to didanosine were up to 12 (rats) and 14.2 (rabbits) times the estimated human exposure at the recommended daily human dose. Didanosine and/or its metabolites are transferred to the fetus through the placenta. In the rat pre/postnatal development study, didanosine administered to pregnant rats reduced food intake and body weight gains in pups at a maternally toxic exposure (approximately 12 times the exposure at the recommended human dose).
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. It is not known whether didanosine is present in human breast milk, affects human milk production, or has effects on the breastfed infant. When administered to lactating rats, didanosine was present in milk (see Data).
Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants) and (3) adverse reactions in breastfed infants similar to those seen in adults, instruct mothers not to breastfeed if they are receiving didanosine delayed-release capsules.
Data
Didanosine and its metabolites were excreted into the milk of lactating rats following a single oral dose of 50 mg per kg on lactation Day 14, with milk concentrations 5 times that of maternal plasma concentrations at 8 and 24 hours post-dose.
8.4 Pediatric Use
Use of didanosine in pediatric patients from 2 weeks of age through adolescence is supported by evidence from adequate and well-controlled studies of didanosine in adult and pediatric patients [see Dosage and Administration (2), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. Additional pharmacokinetic studies in pediatric patients support use of didanosine delayed-release capsules in pediatric patients who weigh at least 20 kg.
8.5 Geriatric Use
In an Expanded Access Program using a buffered formulation of didanosine for the treatment of advanced HIV infection, patients aged 65 years and older had a higher frequency of pancreatitis (10%) than younger patients (5%) [see Warnings and Precautions (5.1)]. Clinical studies of didanosine, including those for didanosine delayed-release capsules, did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. Didanosine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly [see Dosage and Administration (2.2)].
8.6 Renal Impairment
Patients with renal impairment (creatinine clearance of less than 60 mL/min) may be at greater risk of toxicity from didanosine due to decreased drug clearance [see Clinical Pharmacology (12.3)]. A dose reduction is recommended for these patients [see Dosage and Administration (2)].
-
10 OVERDOSAGE
There is no known antidote for didanosine overdosage. In phase 1 studies, in which buffered formulations of didanosine were initially administered at doses ten times the currently recommended dose, toxicities included: pancreatitis, peripheral neuropathy, diarrhea, hyperuricemia, and hepatic dysfunction. Didanosine is not dialyzable by peritoneal dialysis, although there is some clearance by hemodialysis [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Didanosine delayed-release capsules USP are a synthetic purine nucleoside analogue active against HIV-1. Didanosine delayed-release capsules USP, containing enteric-coated beadlets, are available for oral administration in strengths of 125 mg, 200 mg, 250 mg, and 400 mg of didanosine USP. The inactive ingredients in the beadlets include sodium starch glycolate, carboxymethylcellulose sodium, sodium lauryl sulfate, hypromellose, talc, methacrylic acid: ethyl acrylate copolymer (1:1), diethyl phthalate, and colloidal silicon dioxide. In addition, the empty hard gelatin capsule shells also contain gelatin and titanium dioxide. The capsules are printed with edible ink containing black iron oxide and shellac.
The chemical name for didanosine is 2’,3’-dideoxyinosine. The structural formula is:
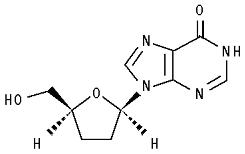
Didanosine USP is a white to almost white, crystalline powder with the molecular formula C10H12N4O3 and a molecular weight of 236.2. The aqueous solubility of didanosine at 25°C and pH of approximately 6 is 27.3 mg/mL. Didanosine is unstable in acidic solutions. For example, at pH less than 3 and 37°C, 10% of didanosine decomposes to hypoxanthine in less than 2 minutes. In didanosine delayed-release capsules USP, an enteric coating is used to protect didanosine from degradation by stomach acid.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
The pharmacokinetic parameters of didanosine in HIV-infected adult and pediatric patients are summarized in Table 7, by weight ranges that correspond to recommended doses (Table 1). Didanosine is rapidly absorbed, with peak plasma concentrations generally observed from 0.25 to 1.5 hours following oral dosing with a buffered formulation. Increases in plasma didanosine concentrations were dose proportional over the range of 50 to 400 mg. In adults, the mean (± standard deviation) oral bioavailability following single oral dosing with a buffered formulation is 42 (±12)%. After oral administration, the urinary recovery of didanosine is approximately 18 (±8)% of the dose. The CSF-plasma ratio following IV administration is 21 (±0.03)%. Steady-state pharmacokinetic parameters did not differ significantly from values obtained after a single dose. Binding of didanosine to plasma proteins in vitro was low (less than 5%). Based on data from in vitro and animal studies, it is presumed that the metabolism of didanosine in man occurs by the same pathways responsible for the elimination of endogenous purines.
Table 7: Pharmacokinetic Parameters for Didanosine in HIV-infected Patients
Pediatrics
Adults
Parametera
20 kg to less than 25 kg
n=10
25 kg to less than 60 kg
n=17
At least 60 kg
n=7
At least 60 kg
n=44
Apparent clearance (L/h)
89.5 ± 21.6
116.2 ± 38.6
196 ± 55.8
174.5 ± 69.7
Apparent volume of distribution (L)
98.1 ± 30.2
154.7 ± 55
363 ± 137.7
308.3 ± 164.3
Elimination half-life (h)
0.75 ± 0.13
0.92 ± 0.09
1.26 ± 0.19
1.19 ± 0.21
Steady-state AUC (mgh/L)
2.38 ± 0.66
2.36 ± 0.7
2.25 ± 0.89
2.65 ± 1.07
a The pharmacokinetic parameters (mean ± standard deviation) of didanosine were determined by a population pharmacokinetic model based on combined clinical studies.
Comparison of Didanosine Formulations
In didanosine delayed-release capsules, the active ingredient, didanosine, is protected against degradation by stomach acid by the use of an enteric coating on the beadlets in the capsule. The enteric coating dissolves when the beadlets empty into the small intestine, the site of drug absorption. With buffered formulations of didanosine, administration with antacid provides protection from degradation by stomach acid.
In healthy volunteers, as well as subjects infected with HIV-1, the AUC is equivalent for didanosine administered as the didanosine delayed-release capsules formulation relative to a buffered tablet formulation. The peak plasma concentration (Cmax) of didanosine, administered as didanosine delayed-release capsules, is reduced approximately 40% relative to didanosine buffered tablets. The time to the peak concentration (Tmax) increases from approximately 0.67 hours for didanosine buffered tablets to 2 hours for didanosine delayed-release capsules.
Effect of Food
In the presence of food, the Cmax and AUC for didanosine delayed-release capsules were reduced by approximately 46% and 19%, respectively, compared to the fasting state [see Dosage and Administration (2)]. Didanosine delayed-release capsules should be taken on an empty stomach.
Special Populations
Renal Insufficiency: Data from two studies using a buffered formulation of didanosine indicated that the apparent oral clearance of didanosine decreased and the terminal elimination half-life increased as creatinine clearance decreased (see Table 8). Following oral administration, didanosine was not detectable in peritoneal dialysate fluid (n=6); recovery in hemodialysate (n=5) ranged from 0.6% to 7.4% of the dose over a 3 to 4 hour dialysis period. The absolute bioavailability of didanosine was not affected in patients requiring dialysis [see Dosage and Administration (2.2)].
Table 8: Mean ± SD Pharmacokinetic Parameters for Didanosine Following a Single Oral Dose of a Buffered Formulation
Parameter
Creatinine Clearance (mL/min)
Dialysis
Patients
n=11
at least 90 n=12
60-90
n=6
30-59
n=6
10-29
n=3
CLcr (mL/min)
112 ± 22
68 ± 8
46 ± 8
13 ± 5
ND
CL/F (mL/min)
2164 ± 638
1566 ± 833
1023 ± 378
628 ± 104
543 ± 174
CLR (mL/min)
458 ± 164
247 ± 153
100 ± 44
20 ± 8
less than 10
T½ (h)
1.42 ± 0.33
1.59 ± 0.13
1.75 ± 0.43
2 ± 0.3
4.1 ± 1.2
ND = not determined due to anuria.
CLcr = creatinine clearance.
CL/F = apparent oral clearance.
CLR= renal clearance.
Hepatic Impairment: The pharmacokinetics of didanosine have been studied in 12 non-HIV- infected subjects with moderate (n=8) to severe (n=4) hepatic impairment (Child-Pugh Class B or C). Mean AUC and Cmax values following a single 400 mg dose of didanosine were approximately 13% and 19% higher, respectively, in patients with hepatic impairment compared to matched healthy subjects. No dose adjustment is needed, because a similar range and distribution of AUC and Cmax values was observed for subjects with hepatic impairment and matched healthy controls [see Dosage and Administration (2.3)].
Pediatric Patients: The pharmacokinetics of didanosine have been evaluated in HIV-exposed and HIV-infected pediatric patients from birth to adulthood.
A population pharmacokinetic analysis was conducted on pooled didanosine plasma concentration data from 9 clinical trials in 106 pediatric (neonate to 18 years of age) and 45 adult patients (greater than 18 years of age). Results showed that body weight is the primary factor associated with oral clearance. Based on the data analyzed, dosing schedule (once versus twice daily) and formulation (powder for oral solution, tablet, and delayed-release capsule) did not have an effect on oral clearance. Didanosine exposure similar to that at recommended adult doses can be achieved in pediatric patients with a weight-based dosing scheme [see Dosage and Administration (2)].
Geriatric Patients: Didanosine pharmacokinetics have not been studied in patients over 65 years of age [see Use in Specific Populations (8.5)].
Gender: The effects of gender on didanosine pharmacokinetics have not been studied.
Drug Interactions
Tables 9 and 10 summarize the effects on AUC and Cmax, with a 90% confidence interval (CI) when available, following coadministration of didanosine delayed-release capsules with a variety of drugs. For clinical recommendations based on drug interaction studies for drugs in bold font [see Dosage and Administration (2.3) and Drug Interactions (7.1)].
Table 9: Results of Drug Interaction Studies with Didanosine Delayed-Release Capsules: Effects of Coadministered Drug on Didanosine Plasma AUC and Cmax Values
% Change of Didanosine
Pharmacokinetic Parametersa
Drug
Didanosine Dosage
n
AUC of Didanosine (90% CI)
Cmax of Didanosine
(90% CI)
tenofovir,b,c 300 mg once daily with a light meald
400 mg single dose fasting 2 hours before tenofovir
26
↑ 48%
(31, 67%)
↑ 48%
(25, 76%)
tenofovir,b,c 300 mg once daily with a light meald
400 mg single dose with tenofovir and a light meal
25
↑ 60%
(44, 79%)
↑ 64%
(41, 89%)
tenofovir,b,c 300 mg once daily with a light meald
200 mg single dose with tenofovir and a light meal
33
↑ 16%
(6, 27%)e
↓ 12%
(-25, 3%)e
250 mg single dose with tenofovir and a light meal
33
↔
(-13, 5%)f
↓ 20%
(-32, -7%)f
325 mg single dose with tenofovir and a light meal
33
↑ 13%
(3, 24%)f
↓ 11%
(-24, 4%)f
methadone, chronic maintenance dose
400 mg single dose
15, 16g
↓ 17%
(-29, -2%)
↓ 16%
(-33, 4%)
↑ Indicates increase.
↓ Indicates decrease.
↔ Indicates no change, or mean increase or decrease of less than 10%.
a The 90% confidence intervals for the percent change in the pharmacokinetic parameter are displayed.
b All studies conducted in healthy volunteers at least 60 kg with creatinine clearance of at least 60 mL/min.
c Tenofovir disoproxil fumarate.
d 373 kilocalories, 8.2 grams fat.
e Compared with didanosine delayed-release capsules 250 mg administered alone under fasting conditions.
f Compared with didanosine delayed-release capsules 400 mg administered alone under fasting conditions.
g Comparisons are made to historical controls (n=148, pooled from 5 studies) conducted in healthy subjects. The number of subjects evaluated for AUC and Cmax is 15 and 16, respectively.
Table 10: Results of Drug Interaction Studies with Didanosine Delayed-Release Capsules: Effects of Didanosine on Coadministered Drug Plasma AUC and Cmax Values
% Change of Coadministered Drug
Pharmacokinetic Parametersa,b
Drug
Didanosine Dosage
n
AUC of Coadministered Drug
(90% CI)
Cmax of Coadministered
Drug
(90% CI)
ciprofloxacin, 750 mg single dose
400 mg single dose
16
↔
↔
indinavir, 800 mg single dose
400 mg single dose
23
↔
↔
ketoconazole, 200 mg single dose
400 mg single dose
21
↔
↔
tenofovir,c 300 mg once daily with a light meald
400 mg single dose fasting 2 hours before tenofovir
25
↔
↔
tenofovir,c 300 mg once daily with a light meald
400 mg single dose with tenofovir and a light meal
25
↔
↔
↔ Indicates no change, or mean increase or decrease of less than 10%.
a The 90% confidence intervals for the percent change in the pharmacokinetic parameter are displayed.
b All studies conducted in healthy volunteers at least 60 kg with creatinine clearance of at least 60 mL/min.
c Tenofovir disoproxil fumarate.
d 373 kilocalories, 8.2 grams fat.
Didanosine Buffered Formulations: Tables 11 and 12 summarize the effects on AUC and Cmax, with a 90% or 95% CI when available, following coadministration of buffered formulations of didanosine with a variety of drugs. The results of these studies may be expected to apply to didanosine delayed-release capsules. For most of the listed drugs, no clinically significant pharmacokinetic interactions were noted. For clinical recommendations based on drug interaction studies for drugs in bold font, [see Dosage and Administration (2.3 for Concomitant Therapy with Tenofovir Disoproxil Fumarate), Contraindications (4), and Drug Interactions (7.1)].
Table 11: Results of Drug Interaction Studies with Buffered Formulations of Didanosine: Effects of Coadministered Drug on Didanosine Plasma AUC and Cmax Values
% Change of Didanosine Pharmacokinetic Parametersa
Drug
Didanosine Dosage
n
AUC of Didanosine
(95% CI)
Cmax of Didanosine
(95% CI)
allopurinol,
renally impaired, 300 mg/day
200 mg single dose
2
↑ 312%
↑ 232%
healthy volunteer, 300 mg/day for 7 days
400 mg single dose
14
↑ 113%
↑ 69%
ganciclovir, 1000 mg every 8 hours, 2 hours after didanosine
200 mg every
12 hours
12
↑ 111%
NA
ciprofloxacin, 750 mg every 12 hours for 3 days, 2 hours before didanosine
200 mg every
12 hours for 3 days
8c
↓ 16%
↓ 28%
indinavir, 800 mg single dose simultaneous
200 mg single dose
16
↔
↔
1 hour before didanosine
200 mg single dose
16
↓ 17%
(-27, -7%)b
↓ 13%
(-28, 5%)b
ketoconazole, 200 mg/day
for 4 days, 2 hours before didanosine
375 mg every
12 hours for 4 days
12c
↔
↓12%
loperamide, 4 mg every
6 hours for 1 day
300 mg single dose
12c
↔
↓23%
metoclopramide,
10 mg single dose
300 mg single dose
12c
↔
↑13%
ranitidine, 150 mg single dose, 2 hours before didanosine
375 mg single dose
12c
↑14%
↑13%
rifabutin, 300 mg or
600 mg/day for 12 days
167 mg or 250 mg every 12 hours for 12 days
11
↑ 13%
(-1, 27%)
↑ 17%
(-4, 38%)
ritonavir, 600 mg every
12 hours for 4 days
200 mg every
12 hours for 4 days
12
↓ 13%
(0, 23%)
↓ 16%
(5, 26%)
stavudine, 40 mg every
12 hours for 4 days
100 mg every
12 hours for 4 days
10
↔
↔
sulfamethoxazole,
1000 mg single dose
200 mg single dose
8c
↔
↔
trimethoprim, 200 mg
single dose
200 mg single dose
8c
↔
↑ 17%
(-23, 77%)
zidovudine, 200 mg every
8 hours for 3 days
200 mg every
12 hours for 3 days
6c
↔
↔
↑ Indicates increase.
↓ Indicates decrease.
↔ Indicates no change, or mean increase or decrease of less than 10%.
a The 95% confidence intervals for the percent change in the pharmacokinetic parameter are displayed.
b 90% CI.
c HIV-infected patients.
NA = Not available.
Table 12: Results of Drug Interaction Studies with Buffered Formulations of Didanosine: Effects of Didanosine on Coadministered Drug Plasma AUC and Cmax Values
% Change of Coadministered Drug Pharmacokinetic Parametersa
Drug
Didanosine Dosage
n
AUC of Coadministered Drug
(95% CI)
Cmax of Coadministered Drug
(95% CI)
dapsone, 100 mg single dose
200 mg every
12 hours for 14 days
6b
↔
↔
ganciclovir, 1000 mg every 8 hours, 2 hours after didanosine
200 mg
every 12 hours
12b
↓21%
NA
nelfinavir, 750 mg single dose, 1 hour after didanosine
200 mg single dose
10b
↑12%
↔
ranitidine, 150 mg single dose, 2 hours before didanosine
375 mg single dose
12b
↓16%
↔
ritonavir, 600 mg every 12 hours for 4 days
200 mg every
12 hours for 4 days
12
↔
↔
stavudine, 40 mg every 12 hours for 4 days
100 mg every
12 hours for 4 days
10b
↔
↑17%
sulfamethoxazole, 1000 mg single dose
200 mg single dose
8b
↓ 11%
(-17, -4%)
↓ 12%
(-28, 8%)
trimethoprim, 200 mg single dose
200 mg single dose
8b
↑ 10%
(-9, 34%)
↓ 22%
(-59, 49%)
zidovudine, 200 mg every 8 hours for 3 days
200 mg every
12 hours for 3 days
6b
↓ 10%
(-27, 11%)
↓ 16.5%
(-53, 47%)
↑ Indicates increase.
↓ Indicates decrease.
↔ Indicates no change, or mean increase or decrease of less than 10%.
a The 95% confidence intervals for the percent change in the pharmacokinetic parameter are displayed.
b HIV-infected patients.
NA = Not available.
12.4 Microbiology
Mechanism of Action
Didanosine is a synthetic nucleoside analogue of the naturally occurring nucleoside deoxyadenosine in which the 3’-hydroxyl group is replaced by hydrogen. Intracellularly, didanosine is converted by cellular enzymes to the active metabolite, dideoxyadenosine 5’-triphosphate. Dideoxyadenosine 5’-triphosphate inhibits the activity of HIV-1 reverse transcriptase both by competing with the natural substrate, deoxyadenosine 5’-triphosphate, and by its incorporation into viral DNA causing termination of viral DNA chain elongation.
Antiviral Activity in Cell Culture
The anti-HIV-1 activity of didanosine was evaluated in a variety of HIV-1 infected lymphoblastic cell lines and monocyte/macrophage cell cultures. The concentration of drug necessary to inhibit viral replication by 50% (EC50) ranged from 2.5 to 10 µM (1 µM = 0.24 mcg/mL) in lymphoblastic cell lines and 0.01 to 0.1 µM in monocyte/macrophage cell cultures.
Resistance
HIV-1 isolates with reduced sensitivity to didanosine have been selected in cell culture and were also obtained from patients treated with didanosine. Genetic analysis of isolates from didanosine-treated patients showed amino acid substitutions K65R, L74V, and M184V in reverse transcriptase. The L74V substitution was most frequently observed in clinical isolates. Phenotypic analysis of HIV-1 isolates from 60 patients (some with prior zidovudine treatment) receiving 6 to 24 months of didanosine monotherapy showed that isolates from 10 of 60 patients exhibited an average of a 10-fold decrease in susceptibility to didanosine in cell culture compared to baseline isolates. Clinical isolates that exhibited a decrease in didanosine susceptibility harbored one or more didanosine resistance-associated substitutions.
Cross-resistance
HIV-1 isolates from 2 of 39 patients receiving combination therapy for up to 2 years with didanosine and zidovudine exhibited decreased susceptibility to didanosine, lamivudine, stavudine, and zidovudine in cell culture. These isolates harbored five substitutions (A62V, V75I, F77L, F116Y, and Q151M) in reverse transcriptase. In data from clinical studies, the presence of thymidine analogue mutation substitutions (M41L, D67N, L210W, T215Y, K219Q) has been shown to decrease the response to didanosine. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were conducted in mice and rats for 22 and 24 months, respectively. In the mouse study, initial doses of 120, 800, and 1200 mg/kg/day for each sex were lowered after 8 months to 120, 210, and 210 mg/kg/day for females and 120, 300, and 600 mg/kg/day for males. The two higher doses exceeded the maximally tolerated dose in females and the high dose exceeded the maximally tolerated dose in males. The low dose in females represented 0.68-fold maximum human exposure and the intermediate dose in males represented 1.7-fold maximum human exposure based on relative AUC comparisons. In the rat study, initial doses were 100, 250, and 1000 mg/kg/day, and the high dose was lowered to 500 mg/kg/day after 18 months. The upper dose in male and female rats represented 3-fold maximum human exposure.
Didanosine induced no significant increase in neoplastic lesions in mice or rats at maximally tolerated doses.
Didanosine was positive in the following genetic toxicology assays: 1) the Escherichia coli tester strain WP2 uvrA bacterial mutagenicity assay; 2) the L5178Y/TK+/- mouse lymphoma mammalian cell gene mutation assay; 3) the in vitro chromosomal aberrations assay in cultured human peripheral lymphocytes; 4) the in vitro chromosomal aberrations assay in Chinese Hamster Lung cells; and 5) the BALB/c 3T3 in vitro transformation assay. No evidence of mutagenicity was observed in an Ames Salmonella bacterial mutagenicity assay or in rat and mouse in vivo micronucleus assays.
Reproduction studies have been performed in rats and rabbits at doses up to 12 and 14 times the estimated human exposure at the recommended daily human dose of didanosine delayed-release capsules, respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to didanosine.13.2 Animal Toxicology and/or Pharmacology
Evidence of a dose-limiting skeletal muscle toxicity has been observed in mice and rats (but not in dogs) following long-term (greater than 90 days) dosing with didanosine at doses that were approximately 1.2 to 12 times the estimated human exposure. The relationship of this finding to the potential of didanosine to cause myopathy in humans is unclear. However, human myopathy has been associated with administration of didanosine and other nucleoside analogues.
-
14 CLINICAL STUDIES
14.1 Adult Patients
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients, with a mean CD4 cell count of 411 cells/mm3 (range 39 to 1105 cells/mm3) and a mean plasma HIV-1 RNA of 4.71 log10 copies/mL (range 2.8 to 5.9 log10 copies/mL) at baseline. Patients were primarily males (72%) and Caucasian (53%) with a mean age of 35 years (range 18 to 73 years). The percentages of patients with HIV-1 RNA less than 400 and less than 50 copies/mL and outcomes of patients through 48 weeks are summarized in Figure 1 and Table 13, respectively.
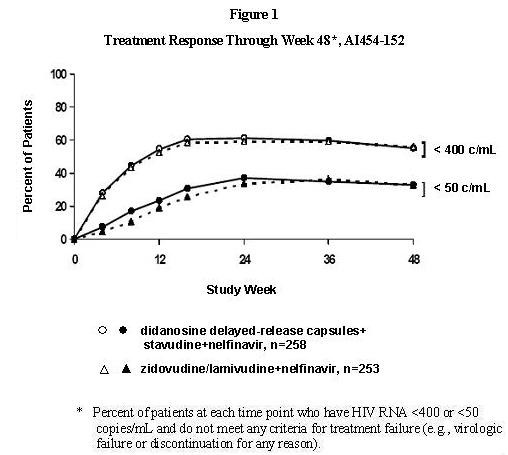
Table 13: Outcomes of Randomized Treatment Through Week 48, AI454-152 Outcome Percent of Patients with HIV-1 RNA less than
400 copies/mL (less than 50 copies/mL)didanosine delayed-release
capsules + stavudine
+ nelfinavir
n=258zidovudine/lamivudinea
+ nelfinavir
n=253a Zidovudine/lamivudine combination tablet.
b Corresponds to rates at Week 48 in Figure 1.
c Subjects achieved and maintained confirmed HIV-1 RNA less than 400 copies/mL (less than 50 copies/mL) through Week 48.
d Includes viral rebound at or before Week 48 and failure to achieve confirmed HIV-1 RNA less than 400 copies/mL (less than 50 copies/mL) through Week 48.
e Includes lost to follow-up, subject’s withdrawal, discontinuation due to physician’s decision, never treated, and other reasons.
Responderb,c
55% (33%)
56% (33%)
Virologic failured
22% (45%)
21% (43%)
Death or discontinued due to
disease progression
1% (1%)
2% (2%)
Discontinued due to adverse
event
6% (6%)
7% (7%)
Discontinued due to other
reasonse
16% (16%)
15% (16%)
14.2 Pediatric Patients
Efficacy in pediatric patients was demonstrated in a randomized, double-blind, controlled study (ACTG 152, conducted 1991 to 1995) involving 831 patients 3 months to 18 years of age treated for more than 1.5 years with zidovudine (180 mg/m2 every 6 hours), didanosine (120 mg/m2 every 12 hours), or zidovudine (120 mg/m2 every 6 hours) plus didanosine (90 mg/m2 every 12 hours). Patients treated with didanosine or didanosine plus zidovudine had lower rates of HIV-1 disease progression or death compared with those treated with zidovudine alone.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Didanosine Delayed-Release Capsules USP, 125 mg are white / white size ‘3’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘70’ on white body with black edible ink filled with white to off-white beadlets.
Bottle of 30 NDC: 65862-310-30
Bottle of 500 NDC: 65862-310-05
10 x 14 Unit-dose Capsules NDC: 65862-310-14
Didanosine Delayed-Release Capsules USP, 200 mg are white / white size ‘1’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘69’ on white body with black edible ink filled with white to off-white beadlets.
Bottle of 30 NDC: 65862-311-30
Bottle of 500 NDC: 65862-311-05
10 x 10 Unit-dose Capsules NDC: 65862-311-10
Didanosine Delayed-Release Capsules USP, 250 mg are white / white size ‘0’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘10’ on white body with black edible ink filled with white to off-white beadlets.
Bottle of 30 NDC: 65862-312-30
Bottle of 500 NDC: 65862-312-05
10 x 10 Unit-dose Capsules NDC: 65862-312-10
Didanosine Delayed-Release Capsules USP, 400 mg are white / white size ‘00’ hard gelatin capsules imprinted with ‘D’ on white cap and ‘09’ on white body with black edible ink filled with white to off-white beadlets.
Bottle of 30 NDC: 65862-313-30
Bottle of 500 NDC: 65862-313-05
10 x 5 Unit-dose Capsules NDC: 65862-313-50
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Store in tightly closed containers. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pancreatitis
Inform patients that a serious toxicity of didanosine, used alone and in combination regimens, is pancreatitis, which may be fatal [see Warnings and Precautions (5.1)].
Lactic Acidosis and Severe Hepatomegaly with Steatosis
Inform patients that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Advise pregnant individuals of the potential risks of lactic acidosis syndrome/hepatic steatosis syndrome [see Contraindications (4), Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
Hepatic Toxicity
Inform patients that hepatotoxicity, including fatal hepatic adverse events, has been reported in patients with preexisting liver dysfunction. The safety and efficacy of didanosine delayed-release capsules have not been established in HIV-infected patients with significant underlying liver disease [see Warnings and Precautions (5.3)].
Non-cirrhotic Portal Hypertension
Inform patients that non-cirrhotic portal hypertension has been reported in patients taking didanosine delayed-release capsules, including cases leading to liver transplantation or death [see Warnings and Precautions (5.4)].
Peripheral Neuropathy
Inform patients that peripheral neuropathy, manifested by numbness, tingling, or pain in hands or feet, may develop during therapy with didanosine delayed-release capsules. Instruct patients that peripheral neuropathy occurs with greatest frequency in patients with advanced HIV-1 disease or a history of peripheral neuropathy, and that discontinuation of didanosine delayed-release capsules may be required if toxicity develops [see Warnings and Precautions (5.5)].
Retinal Changes and Optic Neuritis
Inform patients that retinal changes and optic neuritis, which may result in blurred vision, have been reported in adult and pediatric patients. Advise patients to have regular eye exams while taking didanosine delayed-release capsules [see Warnings and Precautions (5.6)].
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.7)].
Lipoatrophy
Inform patient that loss of body fat (e.g., from arms, legs, or face) may occur in individuals receiving antiretroviral therapy including didanosine delayed-release capsules. Monitor patients receiving didanosine delayed-release capsules to monitor for clinical signs and symptoms of lipoatrophy. Patients should be routinely questioned about body changes related to lipoatrophy [see Warnings and Precautions (5.8)].
Drug Interactions
Didanosine delayed-release capsules may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription of non-prescription medication or herbal products, including alcohol, which may exacerbate didanosine delayed-release capsules toxicities. Patients should avoid alcohol with didanosine delayed-release capsules [see Contraindications (4), Drug Interactions (7)].
Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant individuals exposed to didanosine [see Use in Specific Populations (8.1)].
Lactation
Advise mothers with HIV-1 not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
Dosing Information
Instruct patients to swallow the capsule whole on an empty stomach and to not open the capsule.
Instruct patients not to miss a dose but if they do, patients should take didanosine delayed-release capsules as soon as possible. Inform patients that it is important to take didanosine delayed-release capsules on a regular dosing schedule and to avoid missing doses as it can result in development of resistance.
Dispense with Medication Guide available at: www.aurobindousa.com/product-medication-guides -
Medication Guide
Didanosine Delayed-Release Capsules USP
(dye dan' oh seen)
Enteric-Coated Beadlets
What is the most important information I should know about didanosine delayed-release capsules?
Didanosine delayed-release capsules can cause serious side effects, including:
- Inflammation of your pancreas (pancreatitis) can happen in people who take didanosine delayed-release capsules and can lead to death. People who take didanosine delayed-release capsules in combination with the medicine stavudine may be at an increased risk for pancreatitis. Do not take didanosine delayed-release capsules with stavudine.
Call your healthcare provider right away if you have any of the following symptoms of pancreatitis:
- severe stomach (abdomen) pain
- swelling of your stomach
- nausea and vomiting
- fever
- Build-up of an acid in your blood (lactic acidosis). Lactic acidosis can happen in some people who take didanosine delayed-release capsules or similar medicines (nucleoside analogues). Lactic acidosis is a serious medical emergency that can lead to death. There have been deaths reported in pregnant women who get lactic acidosis after taking didanosine delayed-release capsules and stavudine. Do not take didanosine delayed-release capsules with stavudine.
Call your healthcare provider right away if you have any of the following symptoms which could be signs of lactic acidosis:
- feel weak or tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have stomach pain with nausea and vomiting
- feel cold, especially in your arms and legs
- feel dizzy or light-headed
- have a fast or irregular heartbeat
- weight loss
- Severe liver problems, including liver failure, can happen in people who take didanosine delayed-release capsules. Your liver may become large (hepatomegaly), you may develop fat in the liver (steatosis), or you may have high blood pressure in the large vein of the liver (portal hypertension). Severe liver problems can lead to liver transplantation or death in some people taking didanosine delayed-release capsules. Taking didanosine delayed-release capsules with medicines that contain hydroxyurea or stavudine may increase your risk for liver problems.
You may be more likely to get lactic acidosis or severe liver problems if you are a female, are very overweight (obese), or have been taking nucleoside analogue medicines for a long time. Call your healthcare provider right away if you have any of the following symptoms of severe liver problems:
- yellowing of your skin or the white of your eyes (jaundice)
- dark or “tea-colored” urine
- light colored stools (bowel movements)
- loss of appetite
- nausea
- pain, aching, or tenderness on the right side of your stomach area
For more information about side effects, see “What are the possible side effects of didanosine delayed-release capsules?”.
What are didanosine delayed-release capsules?
Didanosine delayed-release capsules are a prescription medicine that is used with other antiretroviral medicines to treat Human Immunodeficiency Virus (HIV)-1 infection.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
Do not take didanosine delayed-release capsules if you take:
- allopurinol
- ribavirin
- stavudine
Before you take didanosine delayed-release capsules, tell your healthcare provider about all of your medical conditions, including if you:
- have or had problems with your pancreas
- have or had kidney problems
- have or had liver problems, including hepatitis
- have or had numbness, tingling, or pain in the hands or feet (peripheral neuropathy)
- are receiving dialysis
- drink alcoholic beverages
- are pregnant or plan to become pregnant. It is not known if didanosine delayed-release capsules will harm your unborn baby.
Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines, including didanosine during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take didanosine delayed-release capsules.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if didanosine can pass into your breast milk and if it could harm your baby.
Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Some medicines interact with didanosine delayed-release capsules. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with didanosine delayed-release capsules.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take didanosine delayed-release capsules with other medicines.
How should I take didanosine delayed-release capsules?
- Take didanosine delayed-release capsules exactly as your healthcare provider tells you to take them.
- Your healthcare provider will tell you how much didanosine to take and when to take it.
- Take didanosine delayed-release capsules on an empty stomach.
- Take didanosine delayed-release capsules whole. If you cannot swallow didanosine delayed-release capsules whole, tell your healthcare provider. You may need a different medicine.
- Your healthcare provider may change your dose. Do not change your dose of didanosine delayed-release capsules without talking to your healthcare provider.
- Do not miss a dose of didanosine delayed-release capsules. If you miss a dose of didanosine delayed-release capsules, take it as soon as possible.
- It is important to take didanosine delayed-release capsules on a regular schedule. The virus in your blood may increase and the virus may become harder to treat if you miss doses
- Your healthcare provider may lower your dosage of didanosine delayed-release capsules if your kidneys are not working well.
- If you take too much didanosine, go to the nearest emergency room right away.
What are the possible side effects of didanosine delayed-release capsules?
Didanosine delayed-release capsules can cause serious side effects, including:
- See “What is the most important information I should know about didanosine delayed-release capsules?”
- Numbness, tingling, or pain in your hands or feet (peripheral neuropathy). Peripheral neuropathy is common during treatment with didanosine delayed-release capsules and can be severe. Peripheral neuropathy happens more often in people who have advanced HIV-1 disease, have a history of peripheral neuropathy, or in people who are being treated with medicines that can cause neurologic problems. Tell your healthcare provider if you get numbness, tingling, or pain in your hands or feet during treatment with didanosine delayed-release capsules.
- Vision changes. Call your healthcare provider if you have changes in vision, such as blurred vision. You should have regular eye exams while taking didanosine delayed-release capsules.
- Changes in your immune system (immune reconstitution syndrome). Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider if you start having new or worse symptoms of infection after you start taking HIV medicine.
- Loss of body fat (lipoatrophy) can happen with didanosine delayed-release capsules use. These changes may include less fat in your legs, arms, face, and buttocks.
The most common side effects of didanosine delayed-release capsules include:
- diarrhea
- nausea
- headache
- rash
- vomiting
These are not all the possible side effects of didanosine delayed-release capsules.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store didanosine delayed-release capsules?
- Store didanosine delayed-release capsules in a tightly closed container between 15º to 30ºC (59º to 86ºF).
Keep didanosine delayed-release capsules and all medicines out of the reach of children.
General information about the safe and effective use of didanosine delayed-release capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use didanosine delayed-release capsules for a condition for which it was not prescribed. Do not give didanosine delayed-release capsules to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about didanosine delayed-release capsules that is written for health professionals.
For more information, call 1-866-850-2876.
What are the ingredients in didanosine delayed-release capsules?
Active Ingredient: didanosine
Inactive Ingredients:
Sodium starch glycolate, carboxymethylcellulose sodium, sodium lauryl sulfate, hypromellose, talc, methacrylic acid: ethyl acrylate copolymer (1:1), diethyl phthalate, and colloidal silicon dioxide. In addition, the empty hard gelatin capsule shells also contain gelatin and titanium dioxide. The capsules are printed with edible ink containing black iron oxide and shellac.
Dispense with Medication Guide available at: www.aurobindousa.com/product-medication-guides
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 038, India
Revised: 02/2019 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg (30 Capsule Bottle)
NDC: 65862-310-30
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
125 mg
PHARMACIST:Dispense the accompanying
Medication Guide to each patient
AUROBINDO 30 Capsules
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg Blister Carton (10 x 14 Unit-dose)
NDC: 65862-310-14
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
125 mg
PHARMACIST:Dispense the accompanying Medication Guide to each patient
AUROBINDO 140 Unit-dose Capsules (10 x 14s)

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg (30 Capsule Bottle)
NDC: 65862-311-30
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
200 mg
PHARMACIST:Dispense the accompanying
Medication Guide to each patient
AUROBINDO 30 Capsules
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg Blister Carton (10 x 10 Unit-dose)
NDC: 65862-311-10
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
200 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient
AUROBINDO 100 Unit-dose Capsules (10 x 10s)
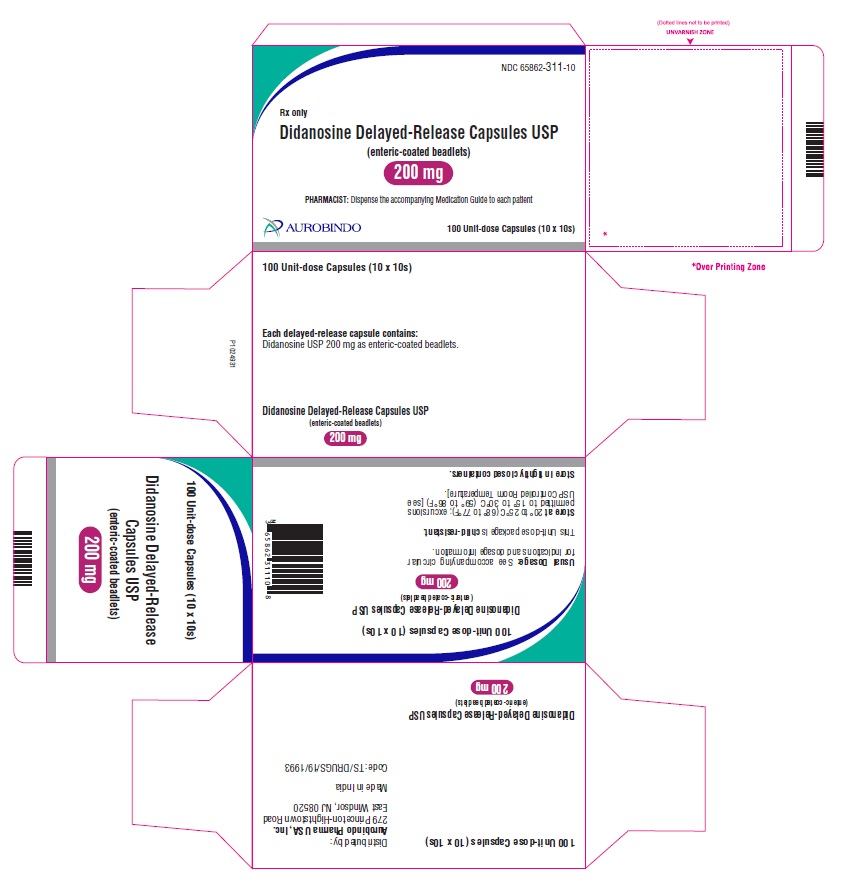
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg (30 Capsule Bottle)
NDC: 65862-312-30
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
250 mg
PHARMACIST:Dispense the accompanying
Medication Guide to each patient
AUROBINDO 30 Capsules

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg Blister Carton (10 x 10 Unit-dose)
NDC: 65862-312-10
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
250 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient
AUROBINDO 100 Unit-dose Capsules (10 x 10s)
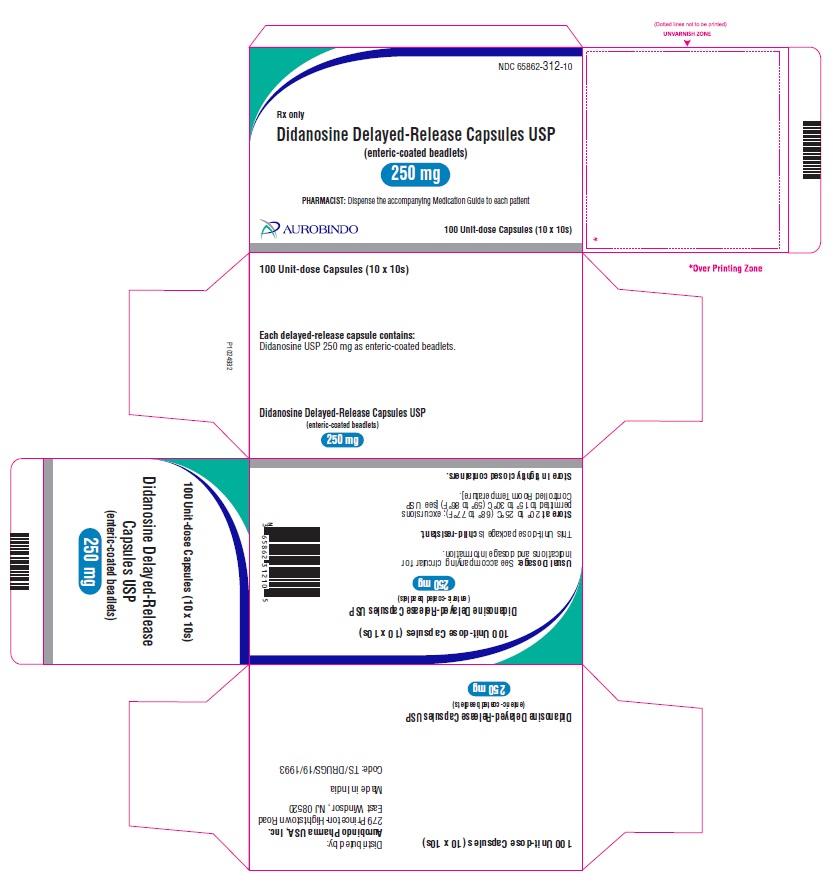
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 400 mg (30 Capsule Bottle)
NDC: 65862-313-30
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
400 mg
PHARMACIST:Dispense the accompanying
Medication Guide to each patient
AUROBINDO 30 Capsules
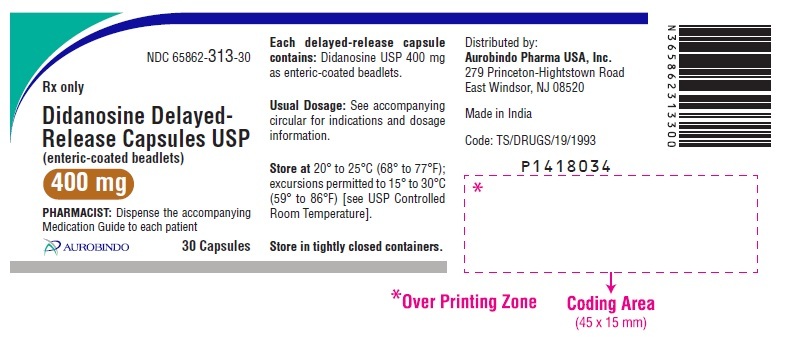
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 400 mg Blister Carton (10 x 5 Unit-dose)
NDC: 65862-313-50
Rx only
Didanosine Delayed-Release Capsules USP
(enteric-coated beadlets)
400 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient
AUROBINDO 50 Unit-dose Capsules (10 x 5s)
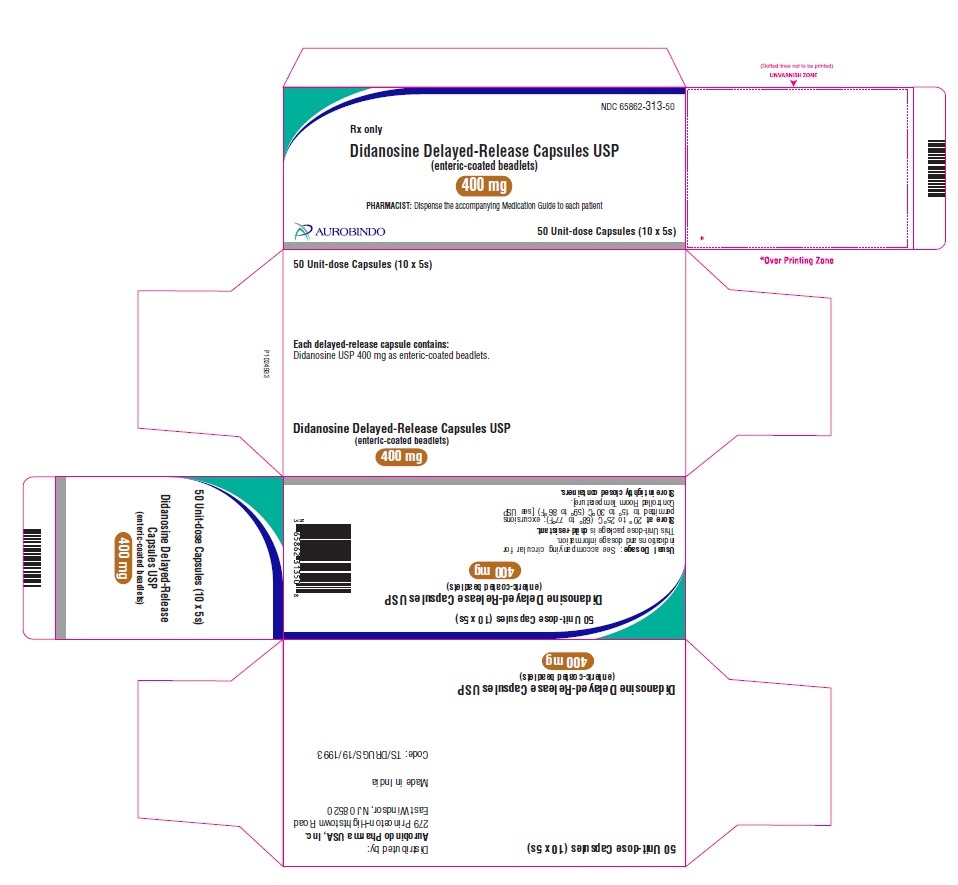
-
INGREDIENTS AND APPEARANCE
DIDANOSINE
didanosine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-310 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIDANOSINE (UNII: K3GDH6OH08) (DIDANOSINE - UNII:K3GDH6OH08) DIDANOSINE 125 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) TALC (UNII: 7SEV7J4R1U) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) DIETHYL PHTHALATE (UNII: UF064M00AF) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code D;70 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-310-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 2 NDC: 65862-310-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 3 NDC: 65862-310-14 10 in 1 CARTON 09/24/2008 3 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090094 09/24/2008 DIDANOSINE
didanosine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-311 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIDANOSINE (UNII: K3GDH6OH08) (DIDANOSINE - UNII:K3GDH6OH08) DIDANOSINE 200 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) TALC (UNII: 7SEV7J4R1U) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) DIETHYL PHTHALATE (UNII: UF064M00AF) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 19mm Flavor Imprint Code D;69 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-311-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 2 NDC: 65862-311-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 3 NDC: 65862-311-10 10 in 1 CARTON 09/24/2008 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090094 09/24/2008 DIDANOSINE
didanosine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-312 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIDANOSINE (UNII: K3GDH6OH08) (DIDANOSINE - UNII:K3GDH6OH08) DIDANOSINE 250 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) TALC (UNII: 7SEV7J4R1U) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) DIETHYL PHTHALATE (UNII: UF064M00AF) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 21mm Flavor Imprint Code D;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-312-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 2 NDC: 65862-312-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 3 NDC: 65862-312-10 10 in 1 CARTON 09/24/2008 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090094 09/24/2008 DIDANOSINE
didanosine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-313 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIDANOSINE (UNII: K3GDH6OH08) (DIDANOSINE - UNII:K3GDH6OH08) DIDANOSINE 400 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) TALC (UNII: 7SEV7J4R1U) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) DIETHYL PHTHALATE (UNII: UF064M00AF) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 24mm Flavor Imprint Code D;09 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-313-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 2 NDC: 65862-313-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2008 3 NDC: 65862-313-50 10 in 1 CARTON 09/24/2008 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090094 09/24/2008 Labeler - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(65862-310, 65862-311, 65862-312, 65862-313) , MANUFACTURE(65862-310, 65862-311, 65862-312, 65862-313)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.