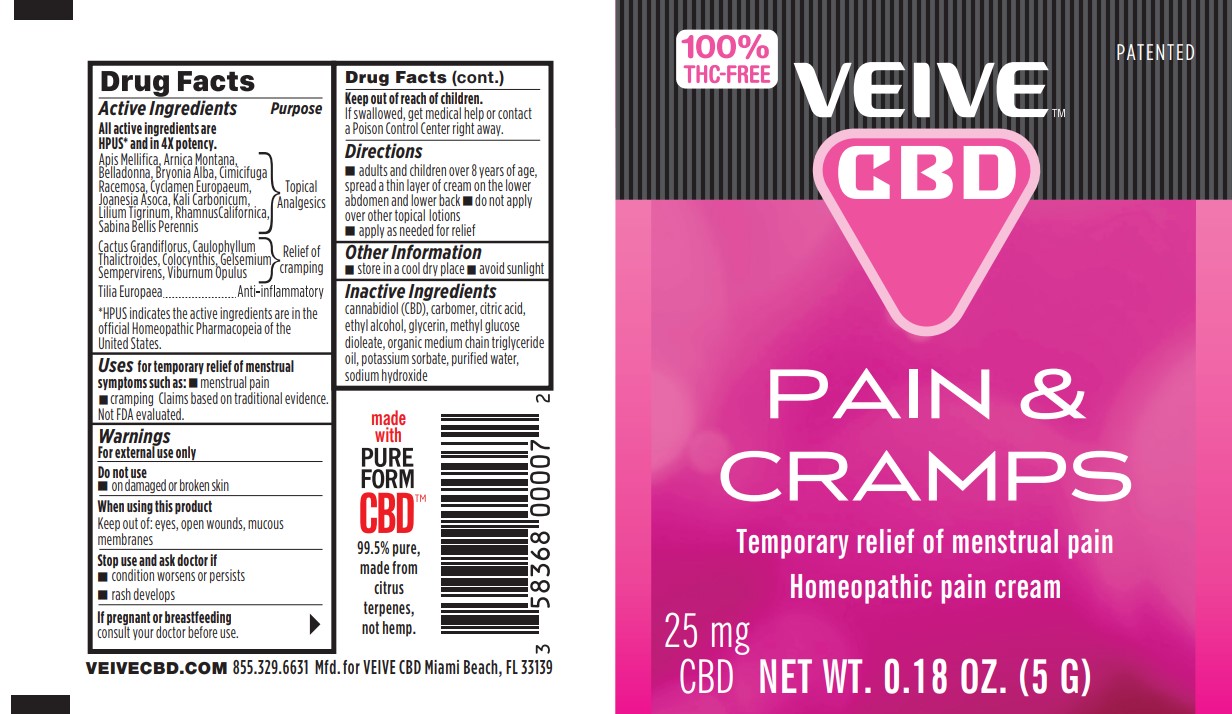
Veive CBD Pain and Cramps by BioLyte Laboratories, LLC
Veive CBD Pain and Cramps by
Drug Labeling and Warnings
Veive CBD Pain and Cramps by is a Homeopathic medication manufactured, distributed, or labeled by BioLyte Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VEIVE CBD PAIN AND CRAMPS- apis mellifica, arnica montana, belladonna, bryonia alba, cimicifuga racemosa, cyclymen europaeum, joanesia asoca, kali carbonicum, lilium tigrinum, rhamnus californica, sabina bellis perennis, cactus grandiflorus, caulophyllum thalictroides, colocynthis, gelsemium sempervirens, viburnum opulus, tilia europaea cream
BioLyte Laboratories, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Active Ingrdeients Purpose
All active ingredients are HPUS* and in 4X potency.
Apis Mellifica, Arnica Montana, Belladonna, Bryonia Alba,
Cimicifuga Racemosa, Cyclamen Europaenum, Joanesia Asoca,
Kali Carbonicum, Lilium Tigrinum, Rhamnus Californica, Sabina,
Bellis Perennis...................................................................................Topical Analgesics
Cactus Grandiflorus, Caulophyllum Thalictroides,
Colocynthis, Gelsemium Sempervirens, Viburnum Opulus................Relief of cramping
Tilia Europaea.....................................................................................Anti-inflammatory
*HPUS indicates the active ingrediens are in the official Homeopathic Pharmacopeia of the United States.
Purpose
Active Ingrdeients Purpose
All active ingredients are HPUS* and in 4X potency.
Apis Mellifica, Arnica Montana, Belladonna, Bryonia Alba,
Cimicifuga Racemosa, Cyclamen Europaenum, Joanesia Asoca,
Kali Carbonicum, Lilium Tigrinum, Rhamnus Californica, Sabina,
Bellis Perennis...................................................................................Topical Analgesics
Cactus Grandiflorus, Caulophyllum Thalictroides,
Colocynthis, Gelsemium Sempervirens, Viburnum Opulus................Relief of cramping
Tilia Europaea.....................................................................................Anti-inflammatory
*HPUS indicates the active ingrediens are in the official Homeopathic Pharmacopeia of the United States.
Uses
Uses for temporary relief of menstrual symptoms such as:
- menstrual pain
- cramping
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Keep our of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions
- adults and children over 8 years of age, spread a thin layer of cream on the lower abdomen and lower back
- do not apply over other topical lotions
- apply as needed for relief
| VEIVE CBD PAIN AND CRAMPS
apis mellifica, arnica montana, belladonna, bryonia alba, cimicifuga racemosa, cyclymen europaeum, joanesia asoca, kali carbonicum, lilium tigrinum, rhamnus californica, sabina bellis perennis, cactus grandiflorus, caulophyllum thalictroides, colocynthis, gelsemium sempervirens, viburnum opulus, tilia europaea cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BioLyte Laboratories, LLC (015560564) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioLyte Laboratories, LLC | 015560564 | manufacture(58368-013) | |