AQUARELLE by INDELPA, S.A DE C.V
AQUARELLE by
Drug Labeling and Warnings
AQUARELLE by is a Otc medication manufactured, distributed, or labeled by INDELPA, S.A DE C.V. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
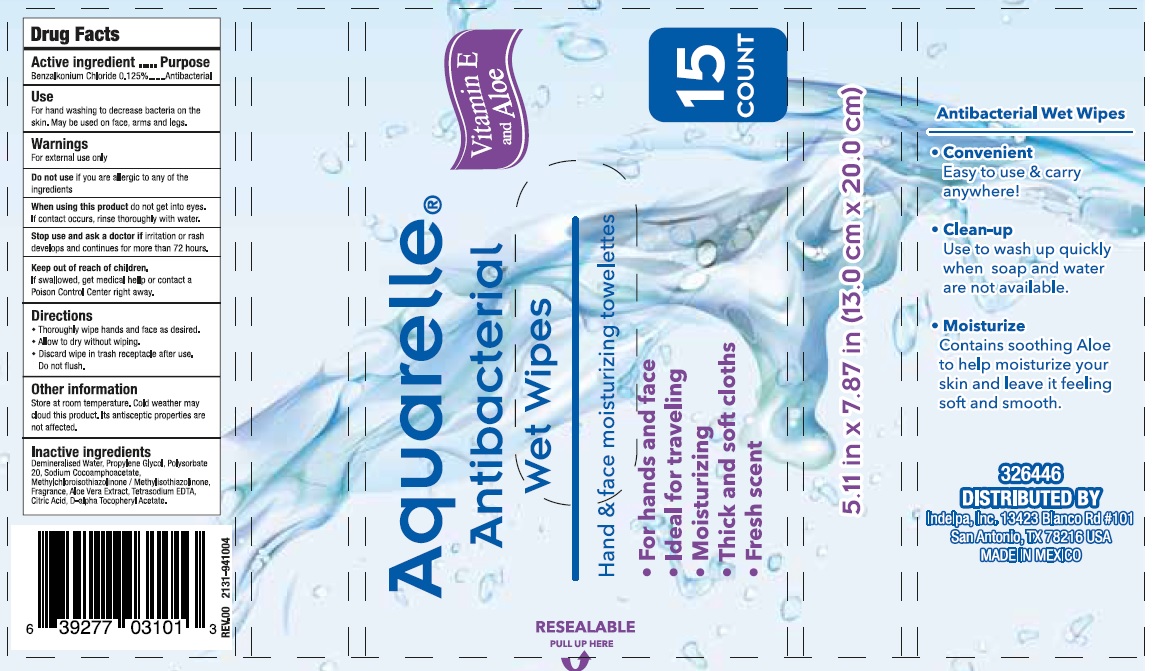
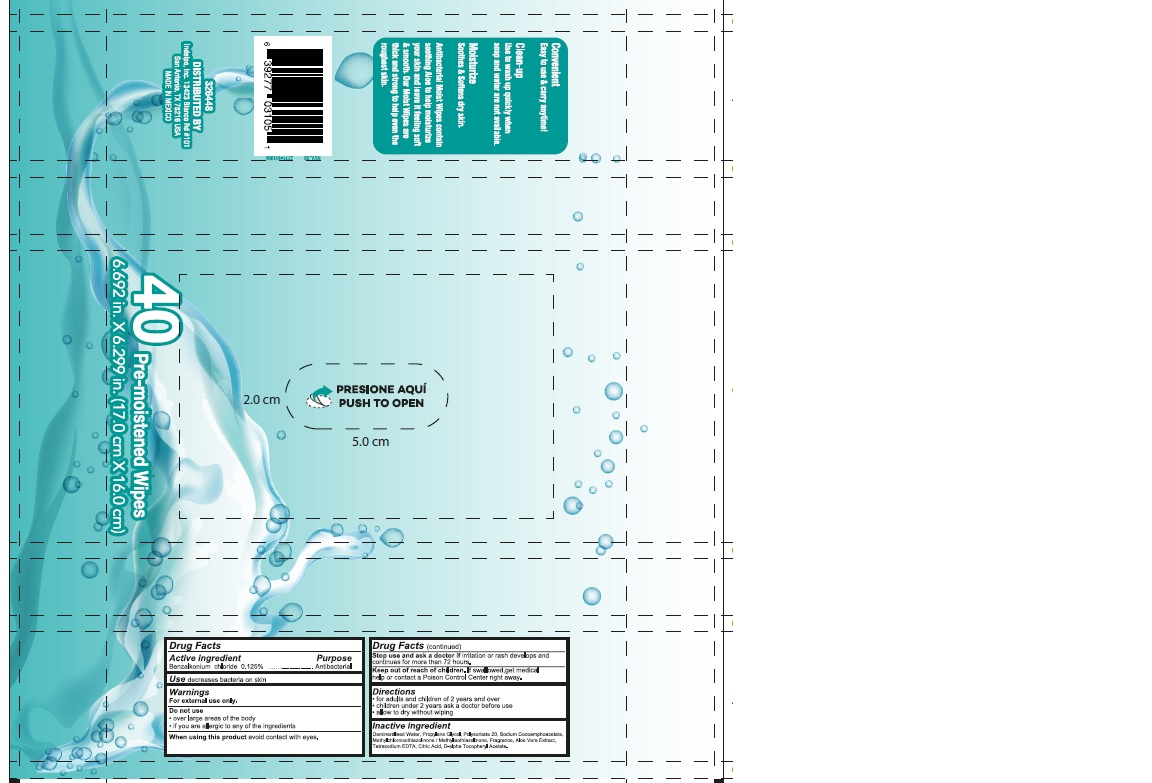
AQUARELLE- benzalkonium chloride swab
INDELPA, S.A DE C.V
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Throughly wipe hands and face as desired.
Allow to dry without wiping.
Discard wipe in trash receptacle after use. Do not flush
Store at room temperature. Cold weather may cloud this product. It´s antisceptic properties are not affected
Demineralised water, Propylene Glycol, Polysorbate 20, Sodium Cocoamphoacetate, Methylchloroisothiazolinone / Methylisothiazolinone, Fragrance, Aloe Vera Extract, Tretasodium EDTA, Citric Acid, D-alpha Tocopheryl Acetate.
| AQUARELLE
benzalkonium chloride swab |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - INDELPA, S.A DE C.V (811072487) |
| Registrant - INDELPA, S.A DE C.V (811072487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| INDELPA, S.A DE C.V | 811072487 | manufacture(70697-803) , analysis(70697-803) , pack(70697-803) , label(70697-803) | |
Trademark Results [AQUARELLE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AQUARELLE 90154114 not registered Live/Pending |
INDELPA, S.A. DE C.V. 2020-09-02 |
 AQUARELLE 79138252 not registered Dead/Abandoned |
AQUARELLE (société anonyme) 2013-04-16 |
 AQUARELLE 78033092 not registered Dead/Abandoned |
S.T.S. Overseas Trading LLC 2000-10-31 |
 AQUARELLE 77526680 not registered Dead/Abandoned |
N.M.B. Medical Applications, Ltd 2008-07-20 |
 AQUARELLE 77008921 not registered Dead/Abandoned |
Elements Swim & Active Wear, LLC 2006-09-27 |
 AQUARELLE 76541401 3194764 Dead/Cancelled |
KONINKLIJKE PHILIPS ELECTRONICS N.V. 2003-08-19 |
 AQUARELLE 76431657 2783857 Live/Registered |
AQUARELLE (SOCIETE PAR ACTIONS SIMPLIFIEE) 2002-07-17 |
 AQUARELLE 76431656 2783856 Live/Registered |
Aquarelle (Societe Par Actions Simplifiee) 2002-07-17 |
 AQUARELLE 76431654 2822277 Dead/Cancelled |
Aquarelle (Societe Anonyme) 2002-07-17 |
 AQUARELLE 75711750 2576471 Dead/Cancelled |
Howmedica Osteonics Corp. 1999-05-21 |
 AQUARELLE 75397678 2238483 Dead/Cancelled |
SHARKEY, JIM 1997-11-19 |
 AQUARELLE 74561962 2018628 Dead/Cancelled |
MERCEDES-BENZ AKTIENGESELLSCHAFT 1994-08-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.