INFANTS PAIN RELIEF- acetaminophen suspension
Infants Pain Relief by
Drug Labeling and Warnings
Infants Pain Relief by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LLC, Aurohealth LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
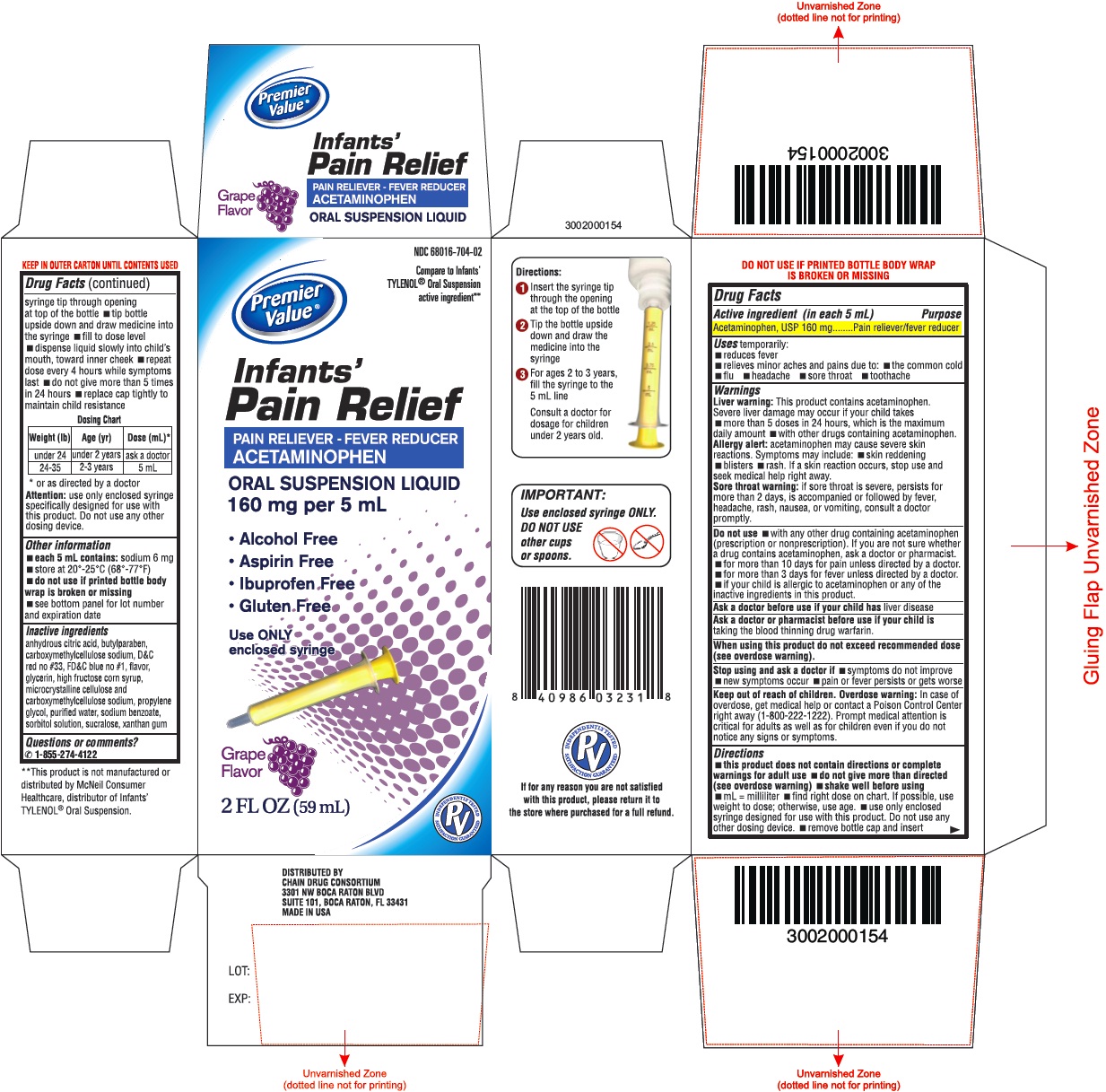
- Drug Facts
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen.
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
-
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor.
- for more than 3 days for fever unless directed by a doctor.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product.
- Ask a doctor before use if your child has
- Ask a doctor or pharmacist before use if your child is
- When using this product
- Stop using and ask a doctor if
- Keep out of reach of children.
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed (see overdose warning)
- shake well before using
- mL = milliliter
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
- use only enclosed syringe designed for use with this product. Do not use any other dosing device.
- remove bottle cap and insert syringe tip through opening at top of the bottle
- tip bottle upside down and draw medicine into the syringe
- fill to dose level
- dispense liquid slowly into child’s mouth, toward inner cheek
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- replace cap tightly to maintain child resistance
Dosing Chart * or as directed by a doctor Weight (lb)
Age (yr)
Dose (mL)*
under 24
under 2 years
ask a doctor
24-35
2-3 years
5 mL
Attention: use only enclosed syringe specifically designed for use with this product. Do not use any other dosing device.
- Other Information
-
Inactive Ingredients
anhydrous citric acid, butylparaben, carboxymethylcellulose sodium, D&C red no #33, FD&C blue no #1, flavor, glycerin, high fructose corn syrup, microcrystalline cellulose and carboxymethylcellulose sodium, propylene glycol, purified water, sodium benzoate, sorbitol solution, sucralose, xanthan gum
Questions or comments?
1-855-274-4122
DISTRIBUTED BY
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431
MADE IN USA -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 2 FL OZ (59 mL)
NDC: 68016-704-02
Compare to Infants'
TYLENOL® Oral Suspension
active ingredient**
Infants'Pain Relief
PAIN RELIEVER -FEVER REDUCER
ACETAMINOPHEN
ORAL SUSPENSION LIQUID
160 mg per 5 mL- Alcohol Free
- Aspirin Free
- Ibuprofen Free
- Gluten Free
Use ONLY
enclosed syringeGrape
Flavor
2 FL OZ (59 mL)
-
INGREDIENTS AND APPEARANCE
INFANTS PAIN RELIEF
acetaminophen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-704 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLPARABEN (UNII: 3QPI1U3FV8) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GRAPE (UNII: 6X543N684K) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE (purple to reddish purple) Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-704-02 1 in 1 CARTON 04/24/2015 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 04/24/2015 Labeler - Chain Drug Consortium, LLC (101668460) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurohealth LLC 078728447 MANUFACTURE(68016-704)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.