BRAVECTO- fluralaner solution
Bravecto by
Drug Labeling and Warnings
Bravecto by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Caution
-
Description:
Each tube is formulated to provide a minimum dose of 11.4 mg/lb (25 mg/kg) body weight. Each milliliter contains 280 mg of fluralaner.
The chemical name of fluralaner is (±)-4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl]-2-methyl-N-[2-oxo-2-(2,2,2-trifluoroethylamino) ethyl]benzamide. Inactive ingredients: dimethylacetamide, glycofurol, diethyltoluamide, acetone
-
Indications:
Bravecto kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick), and Rhipicephalus sanguineus (brown dog tick)] for 12 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.
Bravecto is also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for 8 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.
-
Dosage and Administration:
Bravecto should be administered topically as a single dose every 12 weeks according to the Dosage Schedule below to provide a minimum dose of 11.4 mg/lb (25 mg/kg) body weight.
Bravecto may be administered every 8 weeks in case of potential exposure to Amblyomma americanum ticks (see Effectiveness).
Dosage Schedule Body Weight Ranges (lb) Fluralaner content
(mg)/tubeTubes Administered - * Dogs over 123.0 lb should be administered the appropriate combination of tubes
4.4 – 9.9 112.5 One >9.9 – 22.0 250 One >22.0 – 44.0 500 One >44.0 – 88.0 1000 One >88.0 – 123.0* 1400 One Step 1: Immediately before use, open the pouch and remove the tube. Hold the tube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counter clockwise one full turn. The cap is designed to stay on the tube for dosing and should not be removed. The tube is open and ready for application when a breaking of the seal is felt.
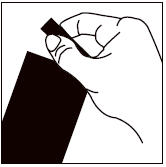

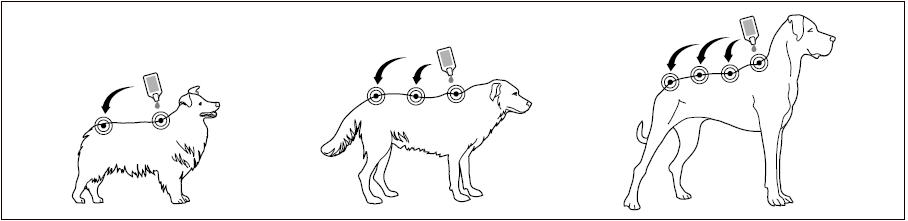
Step 2: The dog should be standing or lying with its back horizontal during application. Part the fur at the administration site. Place the tube tip vertically against the skin between the shoulder blades of the dog.
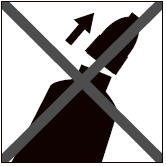
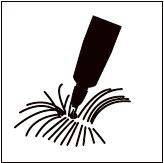
Step 3: Squeeze the tube and gently apply Bravecto in one or more spots starting between the shoulder blades and continuing along the dog's back. Avoid applying an excessive amount of solution in any one spot which may cause some solution to run or drip off of the dog.
Bathing or water immersion 3 days after administration will not reduce the effectiveness of Bravecto against fleas and Ixodes ricinus ticks (see Effectiveness).
Treatment with Bravecto may begin at any time of the year and can continue year round without interruption.
- Contraindications:
-
WARNINGS
Human Warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not contact or allow children to contact the application site until dry.
Keep the product in the original packaging until use in order to prevent children from getting direct access to the product. Do not eat, drink or smoke while handling the product. Avoid contact with skin and eyes. If contact with eyes occurs, then flush eyes slowly and gently with water. Wash hands and contacted skin thoroughly with soap and water immediately after use of the product.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
-
Precautions:
For topical use only. Avoid oral ingestion. (see Animal Safety).
Use with caution in dogs with a history of seizures. Seizures have been reported in dogs receiving fluralaner, even in dogs without a history of seizures (see Adverse Reactions and Animal Safety).
Bravecto has not been shown to be effective for 12-weeks duration in puppies less than 6 months of age. Bravecto is not effective against Amblyomma americanum ticks beyond 8 weeks after dosing (see Effectiveness).
-
Adverse Reactions:
In a well-controlled U.S. field study, which included a total of 165 households and 321 treated dogs (221 with fluralaner and 100 with a topical active control), there were no serious adverse reactions.
Percentage of Dogs with Adverse Reactions in the Field Study Adverse Reaction (AR) Bravecto Group: Percent of Dogs with the AR During the 105-Day Study
(n= 221 dogs)Active Control Group: Percent of Dogs with the AR During the 84-Day Study
(n= 100 dogs)Vomiting 6.3 % 6.0 % Alopecia 4.1 % 2.0 % Diarrhea 2.7 % 11.0 % Lethargy 2.7 % 2.0 % Decreased Appetite 1.4 % 0.0 % Moist Dermatitis/Rash 0.9 % 0.0 % In the field study, two dogs treated with Bravecto with no prior history of seizures each experienced a seizure. One dog had two seizures a day apart about 18 days after its first dose. The dog was started on antiepileptic medication and had no additional seizures during the study. A second dog had a seizure 76 days after its first dose and 3 days after starting fluoxetine for separation anxiety. The fluoxetine was discontinued and the dog experienced no additional seizures during the study. One dog treated with Bravecto was observed by the owner to be off balance for about 30 minutes five days after its first dose and had no similar observations after the second dose. One dog with a history of seizures had a seizure the day after the second dose of the active control.
In two well-controlled laboratory dose confirmation studies, one dog developed mild to moderate redness, flaking, crusts/scabs and alopecia at the treatment site from Day 1 through 14 after application of Bravecto on Day 0, and one dog developed self-limiting generalized erythema (possible allergic reaction) one day after treatment with Bravecto.
In a European field study in cats, there were three reports of facial dermatitis in humans after close contact with the application site which occurred within 4 days of application.
For technical assistance or to report a suspected adverse drug reaction, or to obtain a copy of the Safety Data Sheet (SDS), contact Merck Animal Health at 1-800-224-5318. Additional information can be found at www.bravecto.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
-
Clinical Pharmacology:
Peak fluralaner concentrations are achieved between 7 and 42 days following topical administration and the elimination half-life ranges between 14 and 29 days. The bioavailability of fluralaner following oral and topical administration is approximately 25%.
Mode of Action:
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
-
Effectiveness:
In well-controlled laboratory studies, Bravecto killed fleas and reduced the numbers of live fleas on dogs by >99% and 100% within 24 and 48 hours, respectively, post-infestation for 12 weeks. In well-controlled laboratory studies, Bravecto demonstrated ≥93.3% effectiveness against Dermacentor variabilis, Ixodes scapularis and Rhipicephalus sanguineus 48 hours post-infestation for 12 weeks. Bravecto demonstrated ≥90% effectiveness against Amblyomma americanum 72 hours post-infestation for 8 weeks, but failed to demonstrate ≥90% effectiveness beyond 8 weeks.
In a well-controlled U.S. field study, a single dose of Bravecto reduced fleas by ≥99.8% for 12 weeks. Dogs with signs of flea allergy dermatitis showed improvement in erythema, alopecia, papules, scales, crusts, and excoriation as a direct result of eliminating flea infestations.
In a European laboratory study, Bravecto demonstrated ≥98.2% effectiveness against fleas and Ixodes ricinus ticks after multiple water immersion or shampooing occasions, the first occurring 3 days after application, for 12 weeks.
-
Animal Safety:
Margin of Safety Study: In a margin of safety study, Bravecto was administered topically to 8- to 9-week-old-puppies at 1, 3, and 5× the maximum labeled dose of 56 mg/kg at three, 8-week intervals (8 dogs per group). The dogs in the control group (0×) were administered mineral oil.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Cosmetic changes at the application site included matting/clumping/spiking of hair, wetness, or a greasy appearance.
Oral Safety Study: In a safety study, one dose of Bravecto topical solution was administered orally to 8- to 10-month-old-puppies at 1× the maximum labeled dose of 56 mg/kg. The dogs in the control group (0×) were administered saline orally.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Five of the six treated dogs experienced salivation immediately after administration. One treated animal experienced loose feces with blood three hours after administration. One treated animal experienced vomiting eight hours after administration.
Reproductive Safety: Reproductive safety was evaluated with the oral tablet formulation of fluralaner (see Clinical Pharmacology). Bravecto tablets were administered to intact, reproductively- sound male and female Beagles at a dose of up to 168 mg/kg (equivalent to 3× the maximum treatment dose) on three to four occasions at 8-week intervals. The dogs in the control group (0×) were untreated.
There were no clinically-relevant, treatment-related effects on the body weights, food consumption, reproductive performance, semen analysis, litter data, gross necropsy (adult dogs) or histopathology findings (adult dogs and puppies). One adult treated dog suffered a seizure during the course of the study (46 days after the third treatment). Abnormal salivation was observed on 17 occasions: in six treated dogs (11 occasions) after dosing and four control dogs (6 occasions).
The following abnormalities were noted in 7 pups from 2 of the 10 dams in only the treated group during gross necropsy examination: limb deformity (4 pups), enlarged heart (2 pups), enlarged spleen (3 pups), and cleft palate (2 pups). During veterinary examination at Week 7, two pups from the control group had inguinal testicles, and two and four pups from the treated group had inguinal and cryptorchid testicles, respectively. No undescended testicles were observed at the time of necropsy (days 50 to 71).
In a well-controlled field study Bravecto was used concurrently with other medications, such as vaccines, anthelmintics, antibiotics, steroids and sedatives.
- Storage Information:
- How Supplied:
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 112.5 MG Tube Carton
4.4 – 9.9 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Dogs and Puppies 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 112.5 MG FLURALANER
NADA# 141-459
Approved by FDAx1
MERCK
Animal Health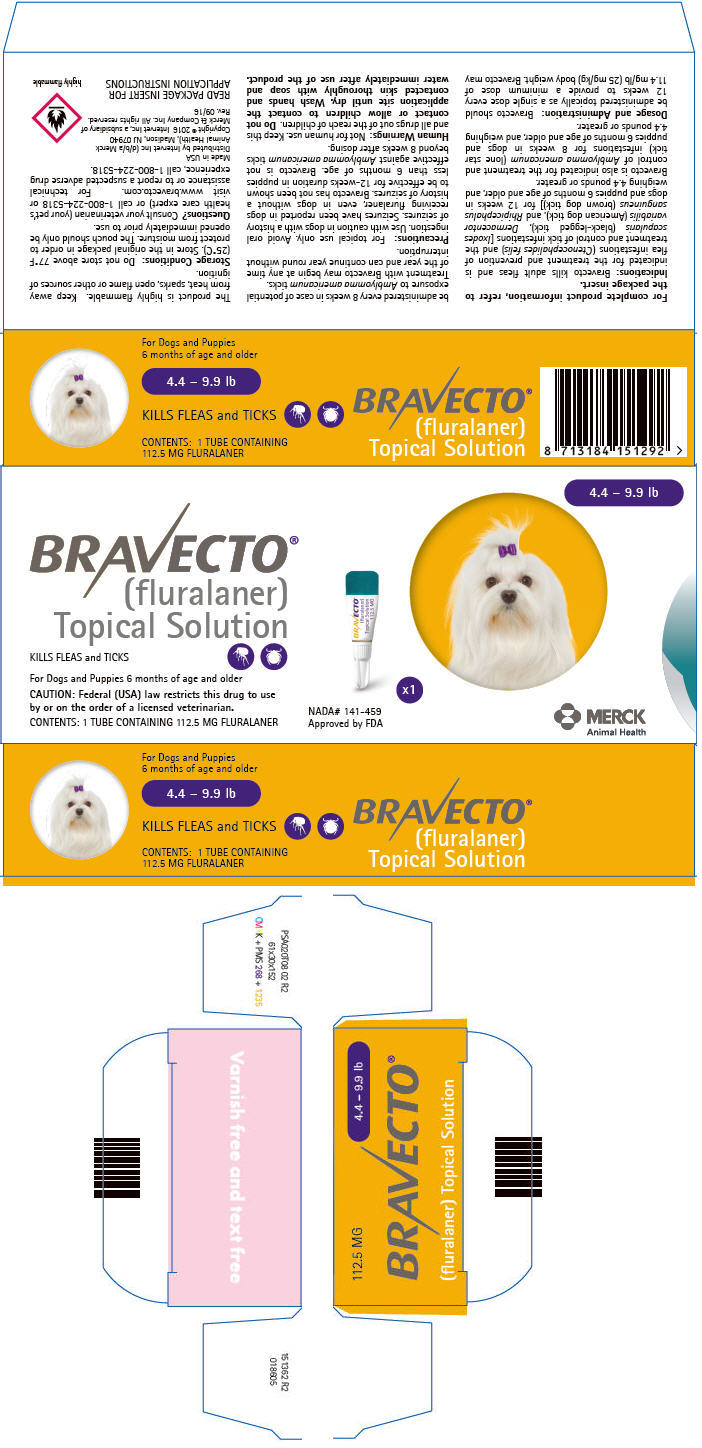
-
PRINCIPAL DISPLAY PANEL - 250 MG Tube Carton
>9.9 – 22 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Dogs and Puppies 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 250 MG FLURALANER
NADA# 141-459
Approved by FDAx1
MERCK
Animal Health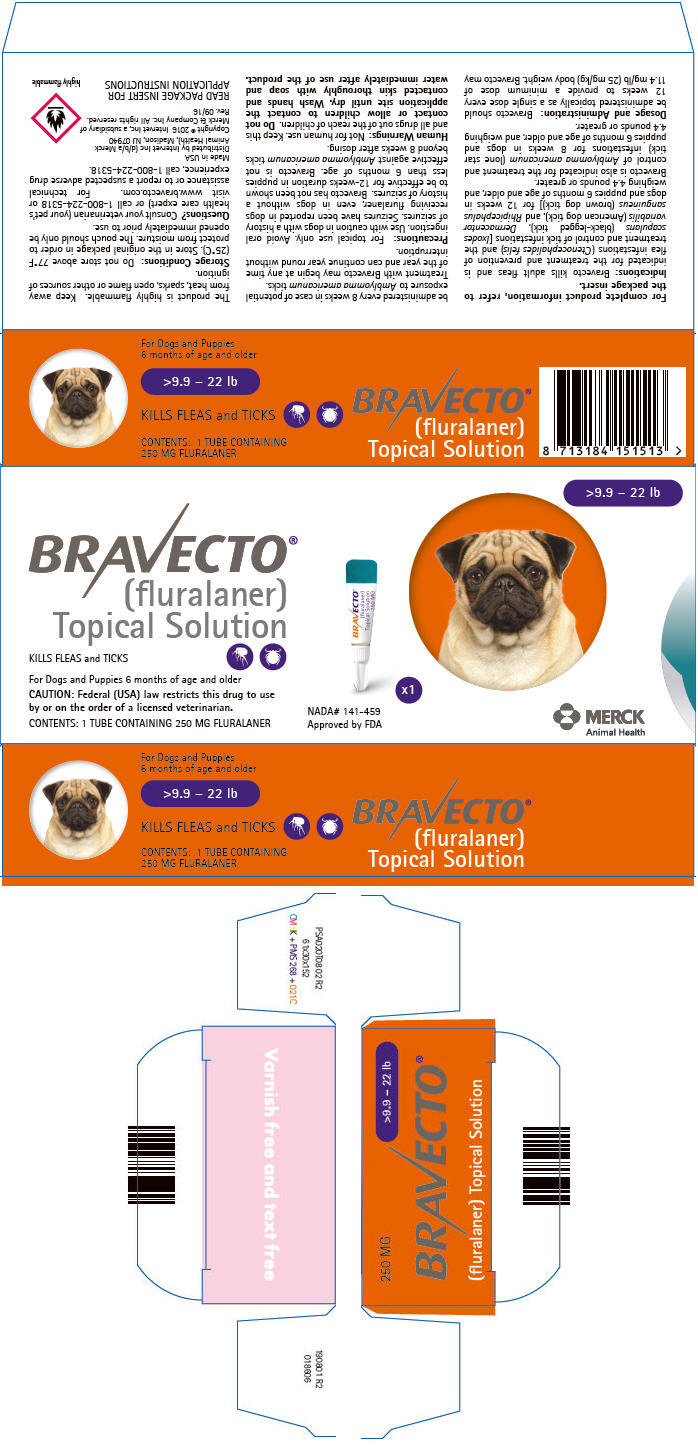
-
PRINCIPAL DISPLAY PANEL - 500 MG Tube Carton
>22 – 44 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Dogs and Puppies 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 500 MG FLURALANER
NADA# 141-459
Approved by FDAx1
MERCK
Animal Health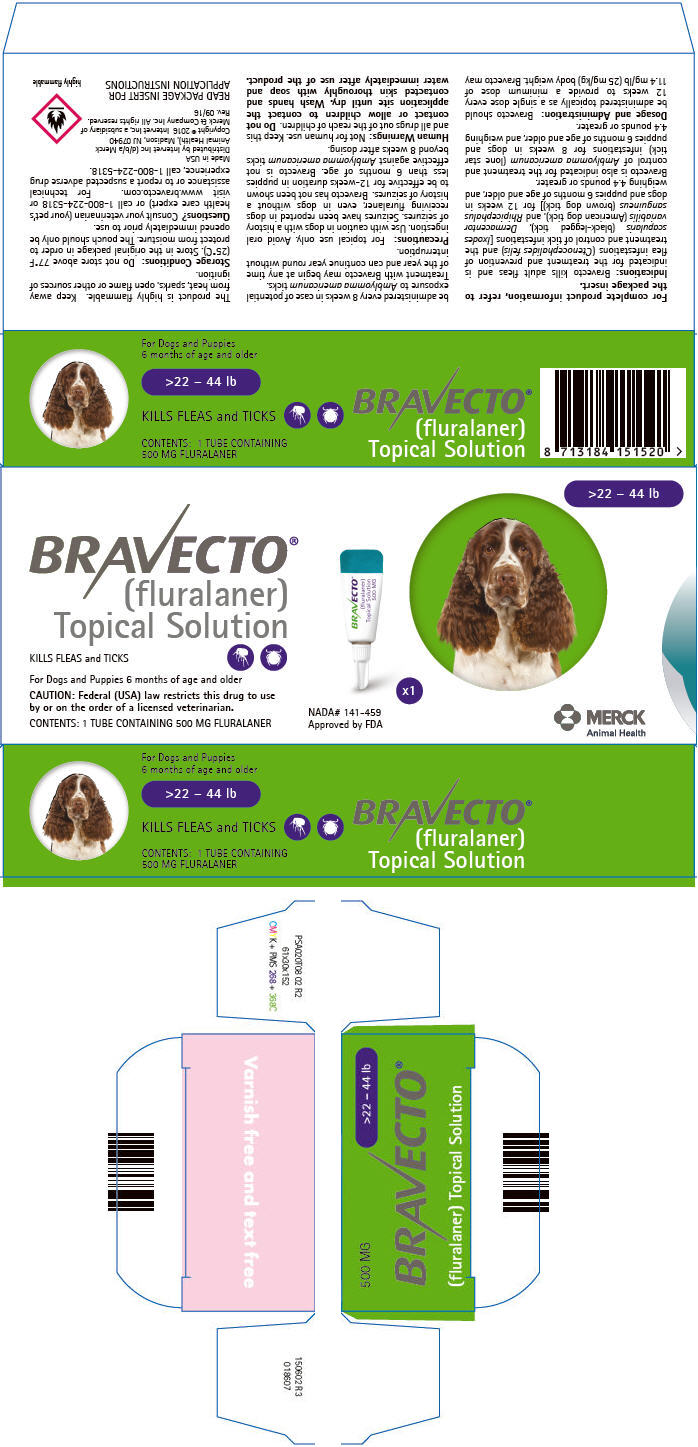
-
PRINCIPAL DISPLAY PANEL - 1000 MG Tube Carton
>44 – 88 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Dogs and Puppies 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 1000 MG FLURALANER
NADA# 141-459
Approved by FDAx1
MERCK
Animal Health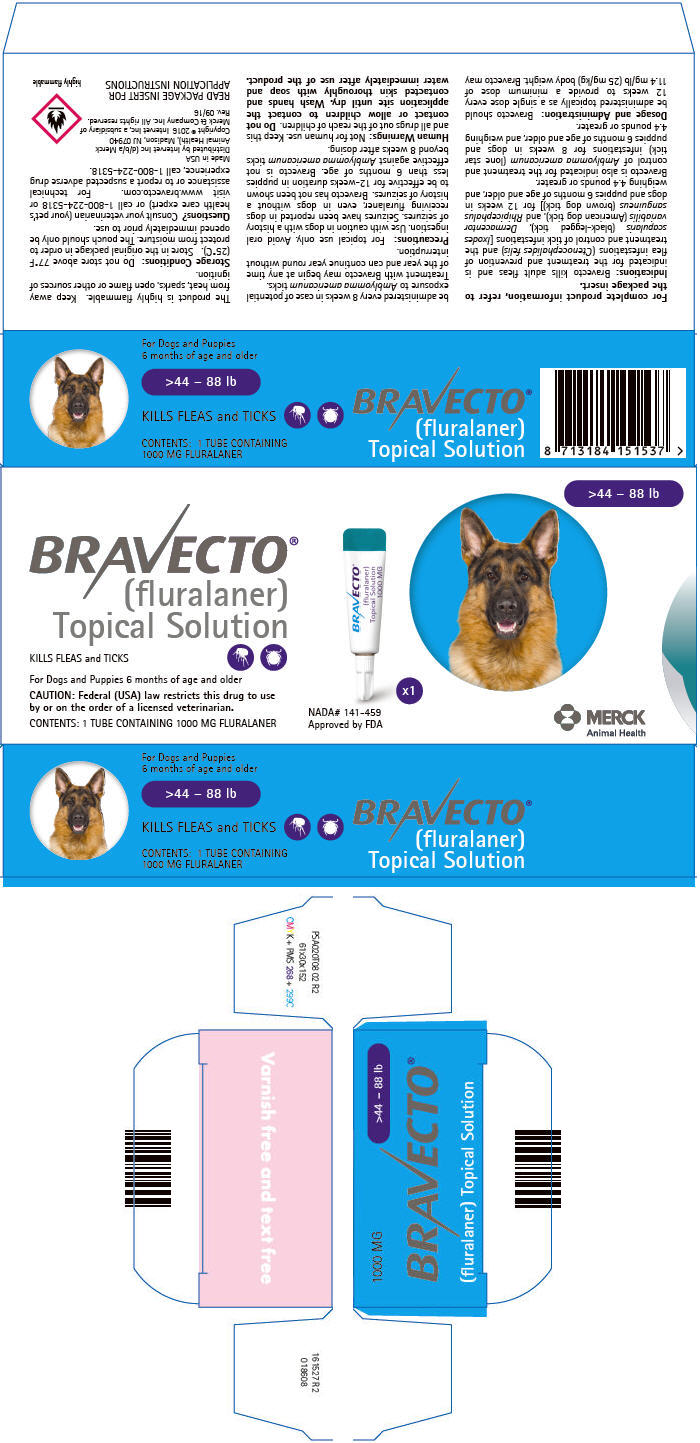
-
PRINCIPAL DISPLAY PANEL - 1400 MG Tube Carton
>88 – 123 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Dogs and Puppies 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 1400 MG FLURALANER
NADA# 141-459
Approved by FDAx1
MERCK
Animal Health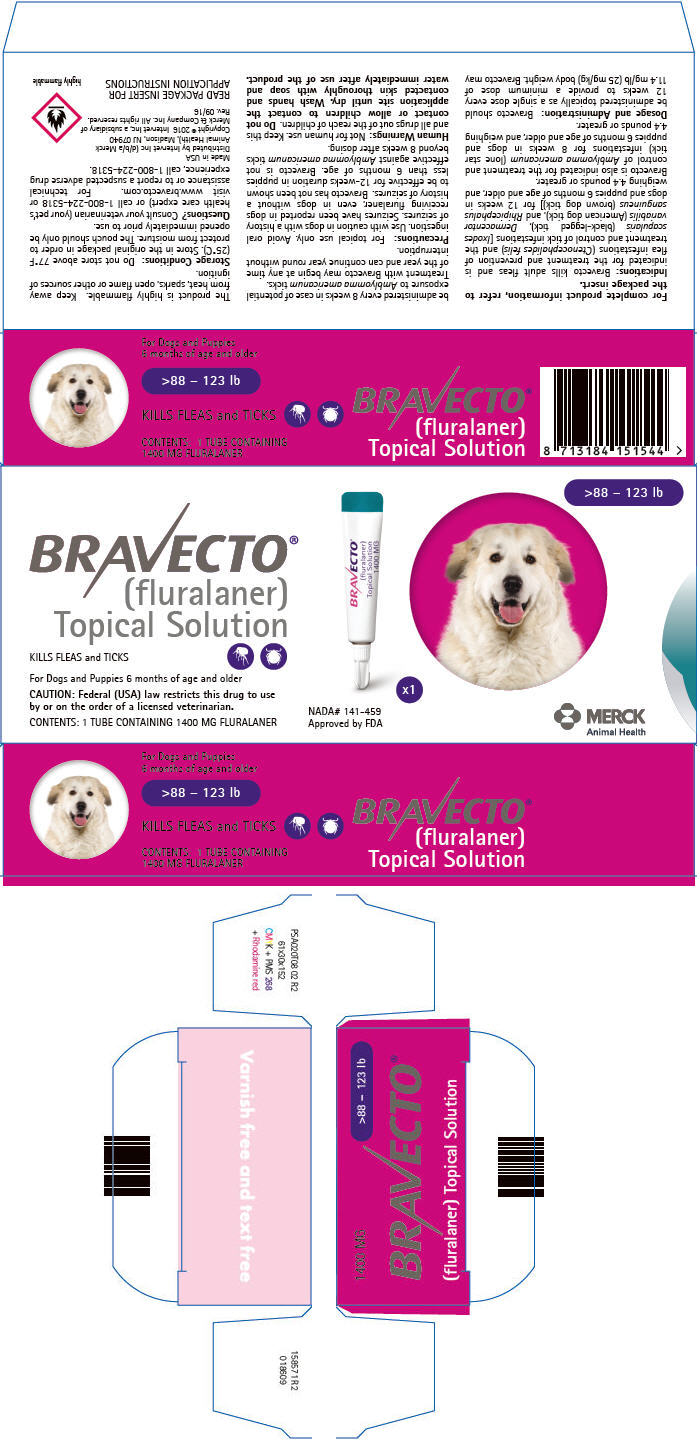
-
INGREDIENTS AND APPEARANCE
BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5500 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 112.5 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5500-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5500-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 250 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5501-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5501-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 500 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5502-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5502-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 1000 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5503-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5503-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5504 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 1400 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5504-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5504-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [Bravecto]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRAVECTO 97938637 not registered Live/Pending |
Intervet Inc. 2023-05-16 |
 BRAVECTO 86307345 4778693 Live/Registered |
Intervet Inc. 2014-06-11 |
 BRAVECTO 85544437 4610171 Live/Registered |
Intervet Inc. 2012-02-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.