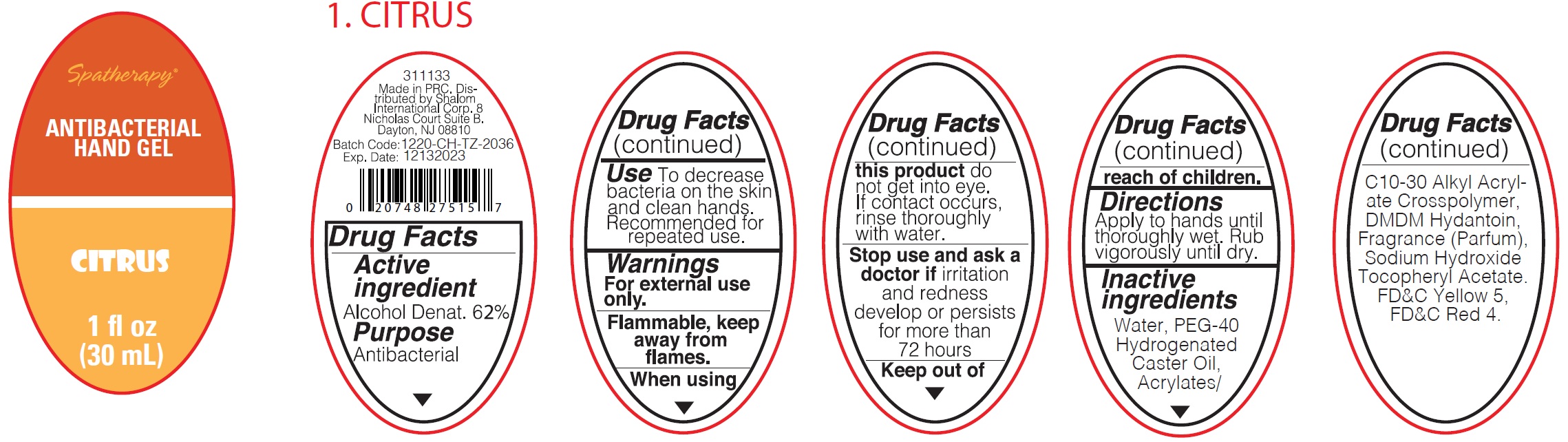
Spatherapy Antibacterial Hand Gel Citrus
Spatherapy Antibacterial Citrus by
Drug Labeling and Warnings
Spatherapy Antibacterial Citrus by is a Otc medication manufactured, distributed, or labeled by Shalom International Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPATHERAPY ANTIBACTERIAL CITRUS- alcohol gel
Shalom International Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Spatherapy Antibacterial Hand Gel Citrus
| SPATHERAPY ANTIBACTERIAL CITRUS
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Shalom International Corp (001384825) |
Revised: 3/2022
Document Id: d9346094-a1ca-7339-e053-2995a90a52bb
Set id: b465e8e9-b2bc-0afb-e053-2a95a90a6299
Version: 2
Effective Time: 20220301