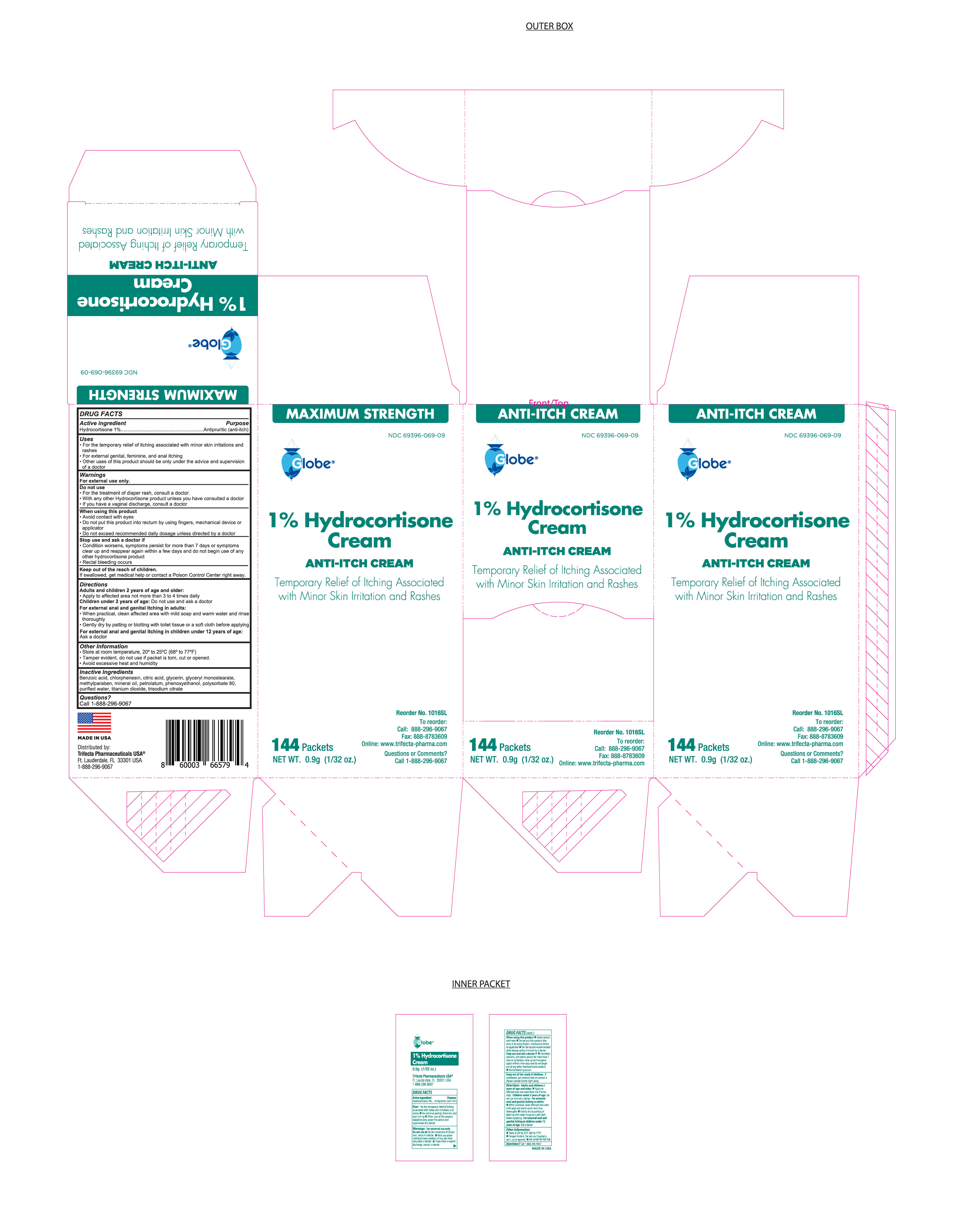
1% Hydrocortisone Cream MAXIMUM STRENGTH
Hydrocortisone 1% by
Drug Labeling and Warnings
Hydrocortisone 1% by is a Otc medication manufactured, distributed, or labeled by Trifecta Pharmaceuticals Usa Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYDROCORTISONE 1%- hydrocortisone cream
Trifecta Pharmaceuticals Usa Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
1% Hydrocortisone Cream
MAXIMUM STRENGTH
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Uses
For temporary relief of itching associated with minor skin irritations and rashes
● external feminine, genital and anal itching
Other uses of this product should be only under the advice and supervision of a doctor
Do Not Use
- For the treatment of diaper rash, consult a doctor.
- With any other Hydrocortisone product unless you have consulted a doctor
- If you have a vaginal discharge, consult a doctor
When using this product
- Avoid Contact with the eyes
- do not exceed the recommended daily dosage unless directed by a doctor.
- do not put this product into the rectum by using fingers or any mechanical device or applicator.
Stop using this product and ask a doctor if
- conditions worsens, symptoms persist for more than 7 days or symptoms clear up and reappear again within a few days and do not begin use of any other hydrocortisone product.
- Rectal bleeding occurs
Directions
Adults and children 2 years of age and older: apply to the affected area not more than 3 to 4 times daily.
Children under 2 years of age: do not use and ask a doctor.
For External and anal itching in adults:
When practical, clean affected area with mild soap and warm water and rinse thoroughly
Gently dry by patting or blotting with toilet tissue or a soft cloth before applying
For External anal and genital itching in children under 12 years of age:
Ask a doctor
Inactive ingredients
Benzoic Acid, Chlorphenesin, citric acid, glycerin, glycerol monostearate, methylparaben, mineral oil, petrolatum, Phenoxyethanol, Polysorbate 80, purified water, titanium dioxide, trisodium citrate
Other information
● store at controlled room temperature 20°-25°C ( 68 °- 77 °F)
Tamper evident, do not use if packet is torn, cut or opened.
Avoid excessive heat and humidity.
| HYDROCORTISONE 1%
hydrocortisone cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Trifecta Pharmaceuticals Usa Llc (079424163) |