Bridge Street Hemp Full Spectrum Pain Relief by BioLyte Laboratories, LLC
Bridge Street Hemp Full Spectrum Pain Relief by
Drug Labeling and Warnings
Bridge Street Hemp Full Spectrum Pain Relief by is a Homeopathic medication manufactured, distributed, or labeled by BioLyte Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BRIDGE STREET HEMP FULL SPECTRUM PAIN RELIEF- atropa belladonna, arnica montana, black cohosh, stellaria media, caulophyllum thalicrtoides root, bryonia alba root, frangula californica bark, hypericum perforatum, rhododendron aureum leaf, rhododendron tomentosum leafy twig cream
BioLyte Laboratories, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Active Ingredients Purpose
All active ingredients are HPUS* & 3X potency.
Belladonna, Bryonia Alba, Caulophyllum,
Cimicifuga Racemosa, Hypericum, Ledum,
Rhamnus Calif., Rhododendron Chrysanthum,
Stellaria Media.........................................................Topical Analgesics
Arnica Montana........................................................Anti-inflammatory
*HPUS indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
Purpose
Active Ingredients Purpose
All active ingredients are HPUS* & 3X potency.
Belladonna, Bryonia Alba, Caulophyllum,
Cimicifuga Racemosa, Hypericum, Ledum,
Rhamnus Calif., Rhododendron Chrysanthum,
Stellaria Media.........................................................Topical Analgesics
Arnica Montana........................................................Anti-inflammatory
*HPUS indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
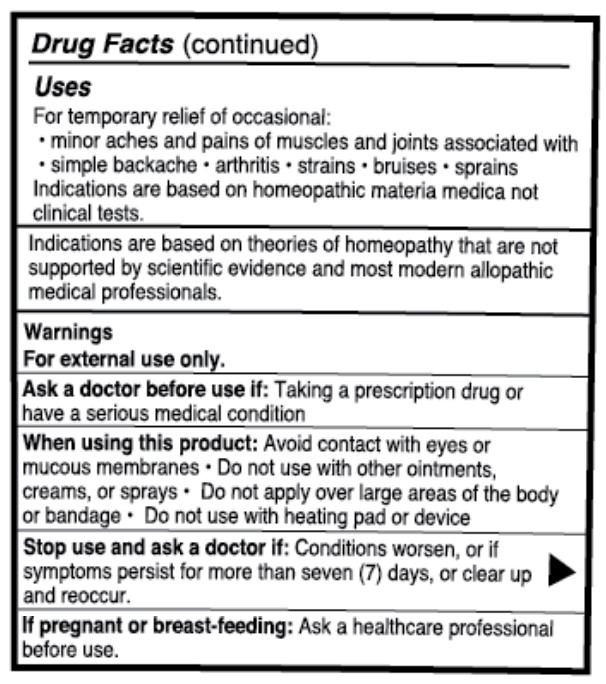
Uses
Uses
For temporary relief of occasional:
- minor aches and pains of muscles and joints associated with
- simple backache
- arthritis
- strains
- bruises
- sprains
Indications are based on homeopathic materia medica not clinical tests.
Indications are based on theories of homeopathy that are not supported by scientific evidence and most modern allopathic medical professionals.
Ask a doctor before use if
Ask a doctor before use if: Taking a prescription drug or have a serious medical condition
When using this product
When using this product: Avoid contact with eyes or mucous membranes
- Do not use with other ointments, creams, or sprays
- Do not apply over large areas of the body or bandage
- do not use with heating pad or device
Stop use and ask a doctor if
Stop use and ask a doctor if: Conditions worsen, or if symptoms persist for more than seven (7) days, or clear up and reoccur.
Stop use and ask a doctor if
Stop use and ask a doctor if: Conditions worsen, or if symptoms persist for more than seven (7) days, or clear up and reoccur.
If pregnant or breast-feeding
If pregnant or breast-feeding: Ask a healthcare professional before use.
Keep out of reach of children
Keep out of reach of children: If accidentally swallowed, get medical help or contact Poison Control Center immediately.
Directions
Directions:
- Adults and children 2 years of age and older, apply a thin layer of gel on clean skin to cover the affected area.
- For best results, allow gel to be aborbed into the skin; do not rub in.
- Apply at the onset of symptoms, or every 15 minutes for the first hour, but not more than 6 times daily.
- Children under 2 years of age: Consult a doctor
Other Information
Other Information
- Store in a cool, dry place.
- Avoid direct sunlight.
- Tamper-evident for your protection. Use only if safety seal is intact.
| BRIDGE STREET HEMP FULL SPECTRUM PAIN RELIEF
atropa belladonna, arnica montana, black cohosh, stellaria media, caulophyllum thalicrtoides root, bryonia alba root, frangula californica bark, hypericum perforatum, rhododendron aureum leaf, rhododendron tomentosum leafy twig cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - BioLyte Laboratories, LLC (015560564) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioLyte Laboratories, LLC | 015560564 | manufacture(58368-018) | |