GANCICLOVIR- ganciclovir sodium injection, powder, lyophilized, for solution
Ganciclovir by
Drug Labeling and Warnings
Ganciclovir by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC , Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
BOXED WARNING
THE CLINICAL TOXICITY OF GANCICLOVIR INCLUDES GRANULOCYTOPENIA, ANEMIA AND THROMBOCYTOPENIA. IN ANIMAL STUDIES GANCICLOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED ASPERMATOGENESIS.
GANCICLOVIR FOR INJECTION IS INDICATED FOR USE ONLY IN THE TREATMENT OF CYTOMEGALOVIRUS (CMV) RETINITIS IN IMMUNOCOMPROMISED PATIENTS AND FOR THE PREVENTION OF CMV DISEASE IN TRANSPLANT PATIENTS AT RISK FOR CMV DISEASE (see INDICATIONS AND USAGE).
-
DESCRIPTION:
Ganciclovir is a synthetic guanine derivative active against cytomegalovirus (CMV).
Ganciclovir for Injection, USP is available as sterile lyophilized powder in strength of 500 mg per vial for intravenous administration only. Each vial of Ganciclovir for Injection, USP contains the equivalent of 500 mg ganciclovir as the sodium salt (46 mg sodium). Reconstitution with 10 mL of Sterile Water for Injection, USP, yields a solution with pH 11 and a ganciclovir concentration of approximately 50 mg/mL. Further dilution in an appropriate intravenous solution must be performed before infusion (see DOSAGE AND ADMINISTRATION).
Ganciclovir is a white to off-white crystalline powder. The chemical name for ganciclovir is 9-[[2-hydroxy-1-(hydroxymethyl)-ethoxy]methyl]guanine. Ganciclovir is a polar hydrophilic compound with a solubility of 2.6 mg/mL in water at 25°C and an n-octanol/water partition coefficient of 0.022. The pKas for ganciclovir are 2.2 and 9.4.
Ganciclovir, when formulated as monosodium salt in the IV dosage form, is a white to off-white lyophilized powder. The chemical name for ganciclovir sodium is 9-[[2-hydroxy-1-hydroxymethyl)-ethoxy]methyl]guanine, monosodium salt. The lyophilized powder has an aqueous solubility of greater than 50 mg/mL at 25°C. At physiological pH, ganciclovir sodium exists as the un-ionized form with a solubility of approximately 6 mg/mL at 37°C.
The structural formulas are as follows:
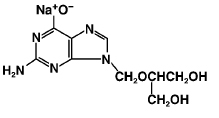
ganciclovir sodium
C9H12N5NaO4 M.W. 277.22
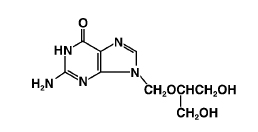
ganciclovir
C9H13N5O4 M.W. 255.23
All doses in this insert are specified in terms of ganciclovir.
-
VIROLOGY:
Mechanism of Action
Ganciclovir is an acyclic nucleoside analogue of 2'-deoxyguanosine that inhibits replication of herpes viruses. Ganciclovir has been shown to be active against cytomegalovirus (CMV) and herpes simplex virus (HSV) in human clinical studies.
To achieve anti-CMV activity, ganciclovir is phosphorylated first to the monophosphate form by a CMV-encoded (UL97 gene) protein kinase homologue, then to the di- and triphosphate forms by cellular kinases. Ganciclovir triphosphate concentrations may be 100-fold greater in CMV-infected than in uninfected cells, indicating preferential phosphorylation in infected cells. Ganciclovir triphosphate, once formed, persists for days in the CMV-infected cell. Ganciclovir triphosphate is believed to inhibit viral DNA synthesis by (1) competitive inhibition of viral DNA polymerases; and (2) incorporation into viral DNA, resulting in eventual termination of viral DNA elongation.
Antiviral Activity
The median concentration of ganciclovir that inhibits CMV replication (IC50) in vitro (laboratory strains or clinical isolates) has ranged from 0.02 to 3.48 mcg/mL. Ganciclovir inhibits mammalian cell proliferation (CIC50) in vitro at higher concentrations ranging from 30 to 725 mcg/mL. Bone marrow-derived colony-forming cells are more sensitive (CIC50 0.028 to 0.7 mcg/mL). The relationship of in vitro sensitivity of CMV to ganciclovir and clinical response has not been established.
Clinical Antiviral Effect of Ganciclovir for Injection and Ganciclovir Capsules
Ganciclovir for Injection
In a study of ganciclovir for injection treatment of life- or sight-threatening CMV disease in immunocompromised patients, 121 of 314 patients had CMV cultured within 7 days prior to treatment and sequential posttreatment viral cultures of urine, blood, throat and/or semen. As judged by conversion to culture negativity, or a greater than 100-fold decrease in in vitro CMV titer, at least 83% of patients had a virologic response with a median response time of 7 to 15 days.
Antiviral activity of ganciclovir for injection was demonstrated in two randomized studies for the prevention of CMV disease in transplant recipients (see Table 1).
Table 1 Patients with Positive CMV Cultures
Heart Allograft* (n=147)
Bone Marrow Allograft (n=72)
Time
Ganciclovir for Injection†
Placebo
Ganciclovir for Injection‡
Placebo
Pretreatment
1/67
(2%)
5/64
(8%)
37/37
(100%)
35/35
(100%)
Week 2
2/75
(3%)
11/67
(16%)
2/31
(6%)
19/28
(68%)
Week 4
3/66
(5%)
28/66
(43%)
0/24
(0%)
16/20
(80%)
* CMV seropositive or receiving graft from seropositive donor.
† 5 mg/kg bid for 14 days followed by 6 mg/kg qd for 5 days/week for 14 days.
‡5 mg/kg bid for 7 days followed by 5 mg/kg qd until day 100 posttransplant.
Ganciclovir Capsules
In trials comparing ganciclovir for injection with ganciclovir capsules for the maintenance treatment of CMV retinitis in patients with AIDS, serial urine cultures and other available cultures (semen, biopsy specimens, blood and others) showed that a small proportion of patients remained culture-positive during maintenance therapy with no statistically significant differences in CMV isolation rates between treatment groups.
Viral Resistance
The current working definition of CMV resistance to ganciclovir in in vitro assays is IC50 >3 mcg/mL (12 mcM). CMV resistance to ganciclovir has been observed in individuals with AIDS and CMV retinitis who have never received ganciclovir therapy. Viral resistance has also been observed in patients receiving prolonged treatment for CMV retinitis with ganciclovir for injection. In a controlled study of oral ganciclovir for prevention of AIDS-associated CMV disease, 364 individuals had one or more cultures performed after at least 90 days of ganciclovir treatment. Of these, 113 had at least one positive culture. The last available isolate from each subject was tested for reduced sensitivity, and 2 of 40 were found to be resistant to ganciclovir. These resistant isolates were associated with subsequent treatment failure for retinitis.
The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy. The principal mechanism of resistance to ganciclovir in CMV is the decreased ability to form the active triphosphate moiety; resistant viruses have been described that contain mutations in the UL97 gene of CMV that controls phosphorylation of ganciclovir. Mutations in the viral DNA polymerase have also been reported to confer viral resistance to ganciclovir.
-
CLINICAL PHARMACOLOGY:
Pharmacokinetics
BECAUSE THE MAJOR ELIMINATION PATHWAY FOR GANCICLOVIR IS RENAL, DOSAGE REDUCTIONS ACCORDING TO CREATININE CLEARANCE ARE REQUIRED FOR GANCICLOVIR FOR INJECTION. FOR DOSING INSTRUCTIONS IN PATIENTS WITH RENAL IMPAIRMENT, REFER TO DOSAGE AND ADMINISTRATION.
Absorption
At the end of a 1-hour intravenous infusion of 5 mg/kg ganciclovir, total AUC ranged between 22.1 ± 3.2 (n=16) and 26.8 ± 6.1 mcg·hr/mL (n=16) and Cmax ranged between 8.27 ± 1.02 (n=16) and 9 ± 1.4 mcg/mL (n=16).
Distribution
The steady-state volume of distribution of ganciclovir after intravenous administration was 0.74 ± 0.15 L/kg (n=98). Cerebrospinal fluid concentrations obtained 0.25 to 5.67 hours postdose in 3 patients who received 2.5 mg/kg ganciclovir intravenously q8h or q12h ranged from 0.31 to 0.68 mcg/mL representing 24% to 70% of the respective plasma concentrations. Binding to plasma proteins was 1% to 2% over ganciclovir concentrations of 0.5 and 51 mcg/mL.
Elimination
When administered intravenously, ganciclovir exhibits linear pharmacokinetics over the range of 1.6 to 5 mg/kg and when administered orally, it exhibits linear kinetics up to a total daily dose of 4 g/day. Renal excretion of unchanged drug by glomerular filtration and active tubular secretion is the major route of elimination of ganciclovir. In patients with normal renal function, 91.3 ± 5% (n=4) of intravenously administered ganciclovir was recovered unmetabolized in the urine. Systemic clearance of intravenously administered ganciclovir was 3.52 ± 0.8 mL/min/kg (n=98) while renal clearance was 3.2 ± 0.8 mL/min/kg (n=47), accounting for 91 ± 11% of the systemic clearance (n=47). Half-life was 3.5 ± 0.9 hours (n=98) following IV administration and 4.8 ± 0.9 hours (n=39) following oral administration.
Special Populations
Renal Impairment
The pharmacokinetics following intravenous administration of ganciclovir for injection solution were evaluated in 10 immunocompromised patients with renal impairment who received doses ranging from 1.25 to 5 mg/kg.
Table 2 Pharmacokinetics of Patients with Renal Impairment
Estimated
Creatinine Clearance
(mL/min)
n
Dose
Clearance
(mL/min)
Mean ± SD
Half-life
(hours)
Mean ± SD
50 to 79
25 to 49
<25
4
3
3
3.2 to 5 mg/kg
3 to 5 mg/kg
1.25 to 5 mg/kg
128 ± 63
57 ± 8
30 ± 13
4.6 ± 1.4
4.4 ± 0.4
10.7 ± 5.7
Based on these observations, it is necessary to modify the dosage of ganciclovir in patients with renal impairment (see DOSAGE AND ADMINISTRATION).
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% after intravenous administration.
Race/Ethnicity and Gender
The effects of race/ethnicity and gender were studied in subjects receiving a dose regimen of 1000 mg every 8 hours. Although the numbers of blacks (16%) and Hispanics (20%) were small, there appeared to be a trend towards a lower steady-state Cmax and AUC 0-8 in these subpopulations as compared to Caucasians. No definitive conclusions regarding gender differences could be made because of the small number of females (12%); however, no differences between males and females were observed.
Pediatrics
Ganciclovir pharmacokinetics were studied in 27 neonates, aged 2 to 49 days. At an intravenous dose of 4 mg/kg (n=14) or 6 mg/kg (n=13), the pharmacokinetic parameters were, respectively, Cmax of 5.5 ± 1.6 and 7 ± 1.6 mcg/mL, systemic clearance of 3.14 ± 1.75 and 3.56 ± 1.27 mL/min/kg, and t1/2 of 2.4 hours (harmonic mean) for both.
Ganciclovir pharmacokinetics were also studied in 10 pediatric patients, aged 9 months to 12 years. The pharmacokinetic characteristics of ganciclovir were the same after single and multiple (q12h) intravenous doses (5 mg/kg). The steady-state volume of distribution was 0.64 ± 0.22 L/kg, Cmax was 7.9 ± 3.9 mcg/mL, systemic clearance was 4.7 ± 2.2 mL/min/kg, and t1/2 was 2.4 ± 0.7 hours. The pharmacokinetics of intravenous ganciclovir in pediatric patients are similar to those observed in adults.
Elderly
No studies have been conducted in adults older than 65 years of age.
-
INDICATIONS AND USAGE:
Ganciclovir for injection is indicated for the treatment of CMV retinitis in immunocompromised patients, including patients with acquired immunodeficiency syndrome (AIDS). Ganciclovir for injection is also indicated for the prevention of CMV disease in transplant recipients at risk for CMV disease (see CLINICAL TRIALS).
SAFETY AND EFFICACY OF GANCICLOVIR FOR INJECTION HAS NOT BEEN ESTABLISHED FOR CONGENITAL OR NEONATAL CMV DISEASE; NOR FOR THE TREATMENT OF ESTABLISHED CMV DISEASE OTHER THAN RETINITIS; NOR FOR USE IN NON-IMMUNOCOMPROMISED INDIVIDUALS.
-
CLINICAL TRIALS:
1. Treatment of CMV Retinitis
The diagnosis of CMV retinitis should be made by indirect ophthalmoscopy. Other conditions in the differential diagnosis of CMV retinitis include candidiasis, toxoplasmosis, histoplasmosis, retinal scars and cotton wool spots, any of which may produce a retinal appearance similar to CMV. For this reason it is essential that the diagnosis of CMV be established by an ophthalmologist familiar with the retinal presentation of these conditions. The diagnosis of CMV retinitis may be supported by culture of CMV from urine, blood, throat or other sites, but a negative CMV culture does not rule out CMV retinitis.
Studies with Ganciclovir for Injection
In a retrospective, non-randomized, single-center analysis of 41 patients with AIDS and CMV retinitis diagnosed by ophthalmologic examination between August 1983 and April 1988, treatment with ganciclovir for injection solution resulted in a significant delay in mean (median) time to first retinitis progression compared to untreated controls [105 (71) days from diagnosis vs 35 (29) days from diagnosis]. Patients in this series received induction treatment of ganciclovir for injection 5 mg/kg bid for 14 to 21 days followed by maintenance treatment with either 5 mg/kg once daily, 7 days per week or 6 mg/kg once daily, 5 days per week (see DOSAGE AND ADMINISTRATION).
In a controlled, randomized study conducted between February 1989 and December 1990,1 immediate treatment with ganciclovir for injection was compared to delayed treatment in 42 patients with AIDS and peripheral CMV retinitis; 35 of 42 patients (13 in the immediate-treatment group and 22 in the delayed-treatment group) were included in the analysis of time to retinitis progression. Based on masked assessment of fundus photographs, the mean [95% CI] and median [95% CI] times to progression of retinitis were 66 days [39, 94] and 50 days [40, 84], respectively, in the immediate-treatment group compared to 19 days [11, 27] and 13.5 days [8, 18], respectively, in the delayed-treatment group.
Studies Comparing Ganciclovir Capsules to Ganciclovir for Injection
Table 3 Population Characteristics in Studies ICM 1653, ICM 1774 and AVI 034
ICM 1653
(n=121)
ICM 1774
(n=225)
AVI 034
(n=159)
Median age (years)
Range
38
24 to 62
37
22 to 56
39
23 to 62
Sex
Males
116 (96%)
222 (99%)
148 (93%)
Females
5 (4%)
3 (1%)
10 (6%)
Ethnicity
Asian
3 (3%)
5 (2%)
7 (4%)
Black
11 (9%)
9 (4%)
3 (2%)
Caucasian
98 (81%)
186 (83%)
140 (88%)
Other
9 (7%)
25 (11%)
8 (5%)
Median CD4 Count Range
9.5
0 to 141
7
0 to 80
10
0 to 320
Mean (SD) Observation Time (days)
107.9 (43)
97.6 (42.5)
80.9 (47)
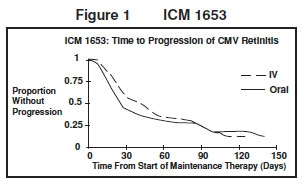
ICM 1653: In this randomized, open-label, parallel group trial, conducted between March 1991 and November 1992, patients with AIDS and newly diagnosed CMV retinitis received a 3-week induction course of ganciclovir for injection solution, 5 mg/kg bid for 14 days followed by 5 mg/kg once daily for 1 additional week.2 Following the 21-day intravenous induction course, patients with stable CMV retinitis were randomized to receive 20 weeks of maintenance treatment with either ganciclovir for injection solution, 5 mg/kg once daily, or ganciclovir capsules, 500 mg 6 times daily (3000 mg/day). The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 57 days [44, 70] and 29 days [28, 43], respectively, for patients on oral therapy compared to 62 days [50, 73] and 49 days [29, 61], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -5 days [-22, 12]. See Figure 1 for comparison of the proportion of patients remaining free of progression over time.
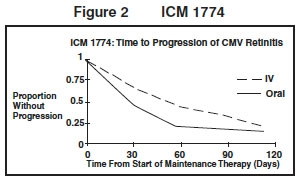
ICM 1774: In this three-arm, randomized, open-label, parallel group trial, conducted between June 1991 and August 1993, patients with AIDS and stable CMV retinitis following from 4 weeks to 4 months of treatment with ganciclovir for injection solution were randomized to receive maintenance treatment with ganciclovir for injection solution, 5 mg/kg once daily, ganciclovir capsules, 500 mg 6 times daily, or ganciclovir capsules, 1000 mg tid for 20 weeks. The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 54 days [48, 60] and 42 days [31, 54], respectively, for patients on oral therapy compared to 66 days [56, 76] and 54 days [41, 69], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -12 days [-24, 0]. See Figure 2 for comparison of the proportion of patients remaining free of progression over time.
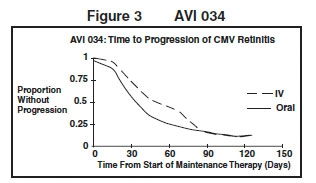
AVI 034: In this randomized, open-label, parallel group trial, conducted between June 1991 and February 1993, patients with AIDS and newly diagnosed (81%) or previously treated (19%) CMV retinitis who had tolerated 10 to 21 days of induction treatment with ganciclovir for injection, 5 mg/kg twice daily, were randomized to receive 20 weeks of maintenance treatment with either ganciclovir capsules, 500 mg 6 times daily or ganciclovir for injection solution, 5 mg/kg/day.3 The mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 51 days [44, 57] and 41 days [31, 45], respectively, for patients on oral therapy compared to 62 days [52, 72] and 60 days [42, 83], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -11 days [-24, 1]. See Figure 3 for comparison of the proportion of patients remaining free of progression over time.
Comparison of other CMV retinitis outcomes between oral and IV formulations (development of bilateral retinitis, progression into Zone 1, and deterioration of visual acuity), while not definitive, showed no marked differences between treatment groups in these studies. Because of low event rates among these endpoints, these studies are underpowered to rule out significant differences in these endpoints.
2. Prevention of CMV Disease in Transplant Recipients
Ganciclovir for injection was evaluated in three randomized, controlled trials of prevention of CMV disease in organ transplant recipients.
ICM 1496: In a randomized, double-blind, placebo-controlled study of 149 heart transplant recipients4 at risk for CMV infection (CMV seropositive or a seronegative recipient of an organ from a CMV seropositive donor), there was a statistically significant reduction in the overall incidence of CMV disease in patients treated with ganciclovir for injection. Immediately posttransplant, patients received ganciclovir for injection solution 5 mg/kg bid for 14 days followed by 6 mg/kg qd for 5 days/week for an additional 14 days. Twelve of the 76 (16%) patients treated with ganciclovir for injection vs 31 of the 73 (43%) placebo-treated patients developed CMV disease during the 120-day post-transplant observation period. No significant differences in hematologic toxicities were seen between the two treatment groups (refer to Table 6 in ADVERSE EVENTS).
ICM 1689: In a randomized, double-blind, placebo-controlled study of 72 bone marrow transplant recipients5 with asymptomatic CMV infection (CMV positive culture of urine, throat or blood) there was a statistically significant reduction in the incidence of CMV disease in patients treated with ganciclovir for injection following successful hematopoietic engraftment. Patients with virologic evidence of CMV infection received ganciclovir for injection solution 5 mg/kg bid for 7 days followed by 5 mg/kg qd through day 100 posttransplant. One of the 37 (3%) patients treated with ganciclovir for injection vs 15 of the 35 (43%) placebo-treated patients developed CMV disease during the study. At 6 months posttransplant, there continued to be a statistically significant reduction in the incidence of CMV disease in patients treated with ganciclovir for injection. Six of 37 (16%) patients treated with ganciclovir for injection vs 15 of the 35 (43%) placebo-treated patients developed disease through 6 months posttransplant. The overall rate of survival was statistically significantly higher in the group treated with ganciclovir for injection, both at day 100 and day 180 posttransplant. Although the differences in hematologic toxicities were not statistically significant, the incidence of neutropenia was higher in the group treated with ganciclovir for injection (refer to Table 6 in ADVERSE EVENTS).
ICM 1570: A second, randomized, unblinded study evaluated 40 allogeneic bone marrow transplant recipients at risk for CMV disease.6 Patients underwent bronchoscopy and bronchoalveolar lavage (BAL) on day 35 posttransplant. Patients with histologic, immunologic or virologic evidence of CMV infection in the lung were then randomized to observation or treatment with ganciclovir for injection solution (5 mg/kg bid for 14 days followed by 5 mg/kg qd 5 days/week until day 120). Four of 20 (20%) patients treated with ganciclovir for injection and 14 of 20 (70%) control patients developed interstitial pneumonia. The incidence of CMV disease was significantly lower in the group treated with ganciclovir for injection, consistent with the results observed in ICM 1689.
- CONTRAINDICATIONS:
-
WARNINGS:
Hematologic
Ganciclovir for injection should not be administered if the absolute neutrophil count is less than 500 cells/mcL or the platelet count is less than 25,000 cells/mcL. Granulocytopenia (neutropenia), anemia and thrombocytopenia have been observed in patients treated with ganciclovir for injection. The frequency and severity of these events vary widely in different patient populations (see ADVERSE EVENTS).
Ganciclovir for injection should, therefore, be used with caution in patients with pre-existing cytopenias or with a history of cytopenic reactions to other drugs, chemicals or irradiation. Granulocytopenia usually occurs during the first or second week of treatment but may occur at any time during treatment. Cell counts usually begin to recover within 3 to 7 days of discontinuing drug. Colony-stimulating factors have been shown to increase neutrophil and white blood cell counts in patients receiving ganciclovir for injection solution for treatment of CMV retinitis.
Impairment of Fertility
Animal data indicate that administration of ganciclovir causes inhibition of spermatogenesis and subsequent infertility. These effects were reversible at lower doses and irreversible at higher doses (see PRECAUTIONS, Carcinogenesis, Mutagenesis‡ and Impairment of Fertility‡). Although data in humans have not been obtained regarding this effect, it is considered probable that ganciclovir at the recommended doses causes temporary or permanent inhibition of spermatogenesis. Animal data also indicate that suppression of fertility in females may occur.
Teratogenesis
Because of the mutagenic and teratogenic potential of ganciclovir, women of childbearing potential should be advised to use effective contraception during treatment. Similarly, men should be advised to practice barrier contraception during and for at least 90 days following treatment with ganciclovir for injection (see PRECAUTIONS, Pregnancy‡: Category C).
-
PRECAUTIONS:
General
In clinical studies with ganciclovir for injection, the maximum single dose administered was 6 mg/kg by intravenous infusion over 1 hour. Larger doses have resulted in increased toxicity. It is likely that more rapid infusions would also result in increased toxicity (see OVERDOSAGE). Administration of ganciclovir for injection solution should be accompanied by adequate hydration.
Initially reconstituted solutions of ganciclovir for injection have a high pH (pH 11). Despite further dilution in intravenous fluids, phlebitis and/or pain may occur at the site of intravenous infusion. Care must be taken to infuse solutions containing ganciclovir for injection only into veins with adequate blood flow to permit rapid dilution and distribution (see DOSAGE AND ADMINISTRATION).
Since ganciclovir is excreted by the kidneys, normal clearance depends on adequate renal function. IF RENAL FUNCTION IS IMPAIRED, DOSAGE ADJUSTMENTS ARE REQUIRED FOR GANCICLOVIR FOR INJECTION. Such adjustments should be based on measured or estimated creatinine clearance values (see DOSAGE AND ADMINISTRATION).
Information for Patients
All patients should be informed that the major toxicities of ganciclovir are granulocytopenia (neutropenia), anemia and thrombocytopenia and that dose modifications may be required, including discontinuation. The importance of close monitoring of blood counts while on therapy should be emphasized. Patients should be informed that ganciclovir has been associated with elevations in serum creatinine.
Patients should be advised that ganciclovir has caused decreased sperm production in animals and may cause infertility in humans. Women of childbearing potential should be advised that ganciclovir causes birth defects in animals and should not be used during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment with ganciclovir for injection. Similarly, men should be advised to practice barrier contraception during and for at least 90 days following treatment with ganciclovir for injection.
Patients should be advised that ganciclovir causes tumors in animals. Although there is no information from human studies, ganciclovir should be considered a potential carcinogen.
All HIV+ Patients
These patients may be receiving zidovudine. Patients should be counseled that treatment with both ganciclovir and zidovudine simultaneously may not be tolerated by some patients and may result in severe granulocytopenia (neutropenia). Patients with AIDS may be receiving didanosine. Patients should be counseled that concomitant treatment with both ganciclovir and didanosine can cause didanosine serum concentrations to be significantly increased.
HIV+ Patients with CMV Retinitis
Ganciclovir is not a cure for CMV retinitis, and immunocompromised patients may continue to experience progression of retinitis during or following treatment. Patients should be advised to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while being treated with ganciclovir for injection. Some patients will require more frequent follow-up.
Transplant Recipients
Transplant recipients should be counseled regarding the high frequency of impaired renal function in transplant recipients who received ganciclovir for injection solution in controlled clinical trials, particularly in patients receiving concomitant administration of nephrotoxic agents such as cyclosporine and amphotericin B. Although the specific mechanism of this toxicity, which in most cases was reversible, has not been determined, the higher rate of renal impairment in patients receiving ganciclovir for injection solution compared with those who received placebo in the same trials may indicate that ganciclovir for injection played a significant role.
Laboratory Testing
Due to the frequency of neutropenia, anemia and thrombocytopenia in patients receiving ganciclovir for injection (see ADVERSE EVENTS), it is recommended that complete blood counts and platelet counts be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in leukopenia, or in whom neutrophil counts are less than 1000 cells/mcL at the beginning of treatment. Increased serum creatinine levels have been observed in trials evaluating both ganciclovir for injection. Patients should have serum creatinine or creatinine clearance values monitored carefully to allow for dosage adjustments in renally impaired patients (see DOSAGE AND ADMINISTRATION).
Drug Interactions
Didanosine
When the standard intravenous ganciclovir induction dose (5 mg/kg infused over 1 hour every 12 hours) was coadministered with didanosine at a dose of 200 mg orally every 12 hours, the steady-state didanosine AUC0-12 increased 70 ± 40% (range: 3% to 121%, n=11) and Cmax increased 49 ± 48% (range: -28% to 125%). In a separate study, when the standard intravenous ganciclovir maintenance dose (5 mg/kg infused over 1 hour every 24 hours) was coadministered with didanosine at a dose of 200 mg orally every 12 hours, didanosine AUC0-12 increased 50 ± 26% (range: 22% to 110%, n=11) and Cmax increased 36 ± 36% (range: -27% to 94%) over the first didanosine dosing interval. Didanosine plasma concentrations (AUC12-24) were unchanged during the dosing intervals when ganciclovir was not coadministered. Ganciclovir pharmacokinetics were not affected by didanosine. In neither study were there significant changes in the renal clearance of either drug.
Zidovudine
At an oral dose of 1000 mg of ganciclovir every 8 hours, mean steady-state ganciclovir AUC0-8 decreased 17 ± 25% (range: -52% to 23%) in the presence of zidovudine, 100 mg every 4 hours (n=12). Steady-state zidovudine AUC0-4 increased 19 ± 27% (range: -11% to 74%) in the presence of ganciclovir. No drug-drug interaction studies have been conducted with IV ganciclovir and zidovudine.
Since both zidovudine and ganciclovir have the potential to cause neutropenia and anemia, some patients may not tolerate concomitant therapy with these drugs at full dosage.
Probenecid
At an oral dose of 1000 mg of ganciclovir every 8 hours (n=10), ganciclovir AUC0-8 increased 53 ± 91% (range: -14% to 299%) in the presence of probenecid, 500 mg every 6 hours. Renal clearance of ganciclovir decreased 22 ± 20% (range: -54% to -4%), which is consistent with an interaction involving competition for renal tubular secretion. No drug-drug interaction studies have been conducted with IV ganciclovir and probenecid.
Imipenem-cilastatin
Generalized seizures have been reported in patients who received ganciclovir and imipenem-cilastatin. These drugs should not be used concomitantly unless the potential benefits outweigh the risks.
Other Medications
It is possible that drugs that inhibit replication of rapidly dividing cell populations such as bone marrow, spermatogonia and germinal layers of skin and gastrointestinal mucosa may have additive toxicity when administered concomitantly with ganciclovir. Therefore, drugs such as dapsone, pentamidine, flucytosine, vincristine, vinblastine, adriamycin, amphotericin B, trimethoprim/sulfamethoxazole combinations or other nucleoside analogues, should be considered for concomitant use with ganciclovir only if the potential benefits are judged to outweigh the risks.
No formal drug interaction studies of ganciclovir for injection and drugs commonly used in transplant recipients have been conducted. Increases in serum creatinine were observed in patients treated with ganciclovir for injection plus either cyclosporine or amphotericin B, drugs with known potential for nephrotoxicity (see ADVERSE EVENTS). In a retrospective analysis of 93 liver allograft recipients receiving ganciclovir (5 mg/kg infused over 1 hour every 12 hours) and oral cyclosporine (at therapeutic doses), there was no evidence of an effect on cyclosporine whole blood concentrations.
Carcinogenesis, Mutagenesis‡
Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1000 mg/kg/day (approximately 0.1x and 1.4x, respectively, the mean drug exposure in humans following the recommended intravenous dose of 5 mg/kg, based on area under the plasma concentration curve [AUC] comparisons). At the dose of 1000 mg/kg/day there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the dose of 20 mg/kg/day, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (estimated as 0.01x the human dose based on AUC comparison). Except for histiocytic sarcoma of the liver, ganciclovir-induced tumors were generally of epithelial or vascular origin. Although the preputial and clitoral glands, forestomach and harderian glands of mice do not have human counterparts, ganciclovir should be considered a potential carcinogen in humans.
Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro at concentrations between 50 to 500 and 250 to 2000 mcg/mL, respectively. In the mouse micronucleus assay, ganciclovir was clastogenic at doses of 150 and 500 mg/kg (IV) (2.8 to 10x human exposure based on AUC) but not 50 mg/kg (exposure approximately comparable to the human based on AUC). Ganciclovir was not mutagenic in the Ames Salmonella assay at concentrations of 500 to 5000 mcg/mL.
Impairment of Fertility‡
Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses of 90 mg/kg/day (approximately 1.7x the mean drug exposure in humans following the dose of 5 mg/kg, based on AUC comparisons). Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration of doses ranging from 0.2 to 10 mg/kg. Systemic drug exposure (AUC) at the lowest dose showing toxicity in each species ranged from 0.03 to 0.1x the AUC of the recommended human intravenous dose.
Pregnancy‡: Category C
Ganciclovir has been shown to be embryotoxic in rabbits and mice following intravenous administration and teratogenic in rabbits. Fetal resorptions were present in at least 85% of rabbits and mice administered 60 mg/kg/day and 108 mg/kg/day (2x the human exposure based on AUC comparisons), respectively. Effects observed in rabbits included: fetal growth retardation, embryolethality, teratogenicity and/or maternal toxicity. Teratogenic changes included cleft palate, anophthalmia/microphthalmia, aplastic organs (kidney and pancreas), hydrocephaly and brachygnathia. In mice, effects observed were maternal/fetal toxicity and embryolethality.
Daily intravenous doses of 90 mg/kg administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the month-old male offspring, as well as pathologic changes in the nonglandular region of the stomach (see Carcinogenesis, Mutagenesis‡). The drug exposure in mice as estimated by the AUC was approximately 1.7x the human AUC.
Ganciclovir may be teratogenic or embryotoxic at dose levels recommended for human use. There are no adequate and well-controlled studies in pregnant women. Ganciclovir for injection should be used during pregnancy only if the potential benefits justify the potential risk to the fetus.
‡ Footnote: All dose comparisons presented in the Carcinogenesis, Mutagenesis‡, Impairment of Fertility‡, and Pregnancy‡ subsections are based on the human AUC following administration of a single 5 mg/kg intravenous infusion of ganciclovir for injection as used during the maintenance phase of treatment. Compared with the single 5 mg/kg intravenous infusion, human exposure is doubled during the intravenous induction phase (5 mg/kg bid). The cross-species dose comparisons should be divided by 2 for intravenous induction treatment with ganciclovir for injection.
Nursing Mothers
It is not known whether ganciclovir is excreted in human milk. However, many drugs are excreted in human milk and, because carcinogenic and teratogenic effects occurred in animals treated with ganciclovir, the possibility of serious adverse reactions from ganciclovir in nursing infants is considered likely (see Pregnancy‡: Category C). Mothers should be instructed to discontinue nursing if they are receiving ganciclovir for injection. The minimum interval before nursing can safely be resumed after the last dose of ganciclovir for injection is unknown.
Pediatric Use
SAFETY AND EFFICACY OF GANCICLOVIR FOR INJECTION IN PEDIATRIC PATIENTS HAVE NOT BEEN ESTABLISHED. THE USE OF GANCICLOVIR FOR INJECTION IN THE PEDIATRIC POPULATION WARRANTS EXTREME CAUTION DUE TO THE PROBABILITY OF LONG-TERM CARCINOGENICITY AND REPRODUCTIVE TOXICITY. ADMINISTRATION TO PEDIATRIC PATIENTS SHOULD BE UNDERTAKEN ONLY AFTER CAREFUL EVALUATION AND ONLY IF THE POTENTIAL BENEFITS OF TREATMENT OUTWEIGH THE RISKS.
The spectrum of adverse events reported in 120 immunocompromised pediatric clinical trial participants with serious CMV infections receiving ganciclovir for injection solution were similar to those reported in adults. Granulocytopenia (17%) and thrombocytopenia (10%) were the most common adverse events reported.
Sixteen pediatric patients (8 months to 15 years of age) with life- or sight-threatening CMV infections were evaluated in an open-label, ganciclovir for injection solution, pharmacokinetics study. Adverse events reported for more than one pediatric patient were as follows: hypokalemia (4/16, 25%), abnormal kidney function (3/16, 19%), sepsis (3/16, 19%), thrombocytopenia (3/16, 19%), leukopenia (2/16, 13%), coagulation disorder (2/16, 13%), hypertension (2/16, 13%), pneumonia (2/16, 13%) and immune system disorder (2/16, 13%).
There has been very limited clinical experience using ganciclovir for injection for the treatment of CMV retinitis in patients under the age of 12 years. Two pediatric patients (ages 9 and 5 years) showed improvement or stabilization of retinitis for 23 and 9 months, respectively. These pediatric patients received induction treatment with 2.5 mg/kg tid followed by maintenance therapy with 6 to 6.5 mg/kg once per day, 5 to 7 days per week. When retinitis progressed during once-daily maintenance therapy, both pediatric patients were treated with the 5 mg/kg bid regimen. Two other pediatric patients (ages 2.5 and 4 years) who received similar induction regimens showed only partial or no response to treatment. Another pediatric patient, a 6-year-old with T-cell dysfunction, showed stabilization of retinitis for 3 months while receiving continuous infusions of ganciclovir for injection at doses of 2 to 5 mg/kg/24 hours. Continuous infusion treatment was discontinued due to granulocytopenia.
Eleven of the 72 patients in the placebo-controlled trial in bone marrow transplant recipients were pediatric patients, ranging in age from 3 to 10 years (5 treated with ganciclovir for injection and 6 with placebo). Five of the pediatric patients treated with ganciclovir for injection received 5 mg/kg intravenously bid for up to 7 days; 4 patients went on to receive 5 mg/kg qd up to day 100 posttransplant. Results were similar to those observed in adult transplant recipients treated with ganciclovir for injection. Two of the 6 placebo-treated pediatric patients developed CMV pneumonia vs none of the 5 patients treated with ganciclovir for injection. The spectrum of adverse events in the pediatric group was similar to that observed in the adult patients.
Geriatric Use
The pharmacokinetic profiles of ganciclovir for injection in elderly patients have not been established. Since elderly individuals frequently have a reduced glomerular filtration rate, particular attention should be paid to assessing renal function before and during administration of ganciclovir for injection (see DOSAGE AND ADMINISTRATION).
Clinical studies of ganciclovir for injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Ganciclovir for injection is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly (see Use in Patients with Renal Impairment and DOSAGE AND ADMINISTRATION).
Use in Patients with Renal Impairment
Ganciclovir for injection should be used with caution in patients with impaired renal function because the half-life and plasma/serum concentrations of ganciclovir will be increased due to reduced renal clearance (see DOSAGE AND ADMINISTRATION and ADVERSE EVENTS).
Hemodialysis has been shown to reduce plasma levels of ganciclovir by approximately 50%.
-
ADVERSE EVENTS:
Adverse events that occurred during clinical trials of ganciclovir for injection solution are summarized below, according to the participating study subject population.
Subjects with AIDS
Three controlled, randomized, phase 3 trials comparing ganciclovir for injection and ganciclovir capsules for maintenance treatment of CMV retinitis have been completed. During these trials, ganciclovir for injection or ganciclovir capsules were prematurely discontinued in 9% of subjects because of adverse events. Laboratory data and adverse events reported during the conduct of these controlled trials are summarized below.
Laboratory Data
Table 4 Selected Laboratory Abnormalities in Trials for Treatment of CMV Retinitis
CMV Retinitis Treatment*
Treatment
Ganciclovir
Capsules†
3000 mg/day
Ganciclovir
for Injection‡
5 mg/kg/day
Subjects, number
320
175
Neutropenia:
<500 ANC/mcL
500 to <749
750 to <1000
18%
17%
19%
25%
14%
26%
Anemia:
Hemoglobin:
<6.5 g/dL
6.5 to <8
8 to <9.5
2%
10%
25%
5%
16%
26%
Maximum Serum Creatinine:
≥2.5 mg/dL
≥1.5 to <2.5
1%
12%
2%
14%
* Pooled data from Treatment Studies, ICM 1653, Study ICM 1774 and Study AVI 034
†Mean time on therapy = 91 days, including allowed reinduction treatment periods
‡ Mean time on therapy = 103 days, including allowed reinduction treatment periods
(See CLINICAL TRIALS.)
Adverse Events
The following table shows selected adverse events reported in 5% or more of the subjects in three controlled clinical trials during treatment with either ganciclovir for injection solution (5 mg/kg/day) or ganciclovir capsules (3000 mg/day), and in one controlled clinical trial in which ganciclovir capsules (3000 mg/day).
Table 5 Selected Adverse Events Reported in ≥ 5% of Subjects in Three Randomized Phase 3 Studies Comparing
Ganciclovir Capsules to Ganciclovir for Injection Solution for Maintenance Treatment of CMV Retinitis
Maintenance Treatment
Studies
Body System
Adverse Event
Capsules
(n=326)
IV
(n=179)
Body as a Whole
Fever
Infection
Chills
Sepsis
38%
9%
7%
4%
48%
13%
10%
15%
Digestive System
Diarrhea
Anorexia
Vomiting
41%
15%
13%
44%
14%
13%
Hemic and Lymphatic System
Leukopenia
Anemia
Thrombocytopenia
29%
19%
6%
41%
25%
6%
Nervous System
Neuropathy
8%
9%
Other
Sweating
Pruritus
11%
6%
12%
5%
Catheter Related*
Total Catheter Events
Catheter Infection
Catheter Sepsis
6%
4%
1%
22%
9%
8%
*Some of these events also appear under other body systems.
The following events were frequently observed in clinical trials but occurred with equal or greater frequency in placebo-treated subjects: abdominal pain, nausea, flatulence, pneumonia, paresthesia, rash.
Retinal Detachment
Retinal detachment has been observed in subjects with CMV retinitis both before and after initiation of therapy with ganciclovir. Its relationship to therapy with ganciclovir is unknown. Retinal detachment occurred in 11% of patients treated with ganciclovir for injection solution and in 8% of patients treated with ganciclovir capsules. Patients with CMV retinitis should have frequent ophthalmologic evaluations to monitor the status of their retinitis and to detect any other retinal pathology.
Transplant Recipients
There have been three controlled clinical trials of ganciclovir for injection solution for the prevention of CMV disease in transplant recipients. Laboratory data and adverse events reported during these trials are summarized below.
Laboratory Data
The following table shows the frequency of granulocytopenia (neutropenia) and thrombocytopenia observed:
Table 6 Controlled Trials — Transplant Recipients
Ganciclovir for Injection
Heart Allograft*
Bone Marrow Allograft†
Ganciclovir
for Injection
(n=76)
Placebo
(n=73)
Ganciclovir
for Injection
(n=57)
Control
(n=55)
Neutropenia
Minimum ANC
<500/mcL
Minimum ANC
500 to 1000/mcL
4%
3%
3%
8%
12%
29%
6%
17%
TOTAL ANC
≤1000/mcL
7%
11%
41%
23%
Thrombocytopenia
Platelet count
<25,000/mcL
Platelet count
25,000 to
50,000/mcL
3%
5%
1%
3%
32%
25%
28%
37%
TOTAL Platelet
≤50,000/mcL
8%
4%
57%
65%
* Study ICM 1496. Mean duration of treatment = 28 days
†Study ICM 1570 and ICM 1689. Mean duration of treatment = 45 days
(See CLINICAL TRIALS.)
The following table shows the frequency of elevated serum creatinine values in these controlled clinical trials:
Table 7 Controlled Trials - Transplant Recipients
Ganciclovir for Injection
Heart Allograft
ICM 1496
Bone Marrow Allograft
ICM 1570
Bone Marrow Allograft
ICM 1689
Maximum
Serum
Creatinine
Levels
Ganciclovir for Injection
(n=76)
Placebo
(n=73)
Ganciclovir for Injection
(n=20)
Control
(n=20)
Ganciclovir for Injection
(n=37)
Placebo
(n=35)
Serum
Creatinine
≥2.5 mg/dL
18%
4%
20%
0%
0%
0%
Serum
Creatinine
≥1.5 to
<2.5 mg/dL
58%
69%
50%
35%
43%
44%
In these three trails, patients receiving ganciclovir for injection solution had elevated serum creatinine levels when compared to those receiving placebo. Most patients in these studies also received cyclosporine. The mechanism of impairment of renal function is not known. However, careful monitoring of renal function during therapy with ganciclovir for injection solution is essential, especially for those patients receiving concomitant agents that may cause nephrotoxicity.
General
Other adverse events that were thought to be "probably" or "possibly" related to ganciclovir solution or ganciclovir capsules in controlled clinical studies in either subjects with AIDS or transplant recipients are listed below. These events all occurred in at least 3 subjects.
Body as a Whole: abdomen enlarged, asthenia, chest pain, edema, headache, injection site inflammation, malaise, pain.
Digestive System: abnormal liver function test, aphthous stomatitis, constipation, dyspepsia, eructation.
Hemic and Lymphatic System: pancytopenia.
Respiratory System: cough increased, dyspnea.
Nervous System: abnormal dreams, anxiety, confusion, depression, dizziness, dry mouth, insomnia, seizures, somnolence, thinking abnormal, tremor.
Skin and Appendages: alopecia, dry skin.
Special Senses: abnormal vision, taste perversion, tinnitus, vitreous disorder.
Metabolic and Nutritional Disorders: creatinine increased, SGOT increased, SGPT increased, weight loss.
Cardiovascular System: hypertension, phlebitis, vasodilatation.
Urogenital System: creatinine clearance decreased, kidney failure, kidney function abnormal, urinary frequency.
Musculoskeletal System: arthralgia, leg cramps, myalgia, myasthenia.
The following adverse events reported in patients receiving ganciclovir may be potentially fatal: gastrointestinal perforation, multiple organ failure, pancreatitis and sepsis.
Adverse Events Reported During Postmarketing Experience with Ganciclovir for Injection and Ganciclovir Capsules
The following events have been identified during post-approval use of the drug. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either the seriousness, frequency of reporting, the apparent causal connection or a combination of these factors:
Acidosis, allergic reaction, anaphylactic reaction, arthritis, bronchospasm, cardiac arrest, cardiac conduction abnormality, cataracts, cholelithiasis, cholestasis, congenital anomaly, dry eyes, dysesthesia, dysphasia, elevated triglyceride levels, encephalopathy, exfoliative dermatitis, extrapyramidal reaction, facial palsy, hallucinations, hemolytic anemia, hemolytic uremic syndrome, hepatic failure, hepatitis, hypercalcemia, hyponatremia, inappropriate serum ADH, infertility, intestinal ulceration, intracranial hypertension, irritability, loss of memory, loss of sense of smell, myelopathy, oculomotor nerve paralysis, peripheral ischemia, pulmonary fibrosis, renal tubular disorder, rhabdomyolysis, Stevens-Johnson syndrome, stroke, testicular hypotrophy, Torsades de Pointes, vasculitis, ventricular tachycardia.
-
OVERDOSAGE:
Overdosage with ganciclovir for injection has been reported in 17 patients (13 adults and 4 children under 2 years of age). Five patients experienced no adverse events following overdosage at the following doses: 7 doses of 11 mg/kg over a 3-day period (adult), single dose of 3500 mg (adult), single dose of 500 mg (72.5 mg/kg) followed by 48 hours of peritoneal dialysis (4-month-old), single dose of approximately 60 mg/kg followed by exchange transfusion (18-month-old), 2 doses of 500 mg instead of 31 mg (21-month-old).
Irreversible pancytopenia developed in 1 adult with AIDS and CMV colitis after receiving 3000 mg of ganciclovir for injection solution on each of 2 consecutive days. He experienced worsening GI symptoms and acute renal failure that required short-term dialysis. Pancytopenia developed and persisted until his death from a malignancy several months later. Other adverse events reported following overdosage included: persistent bone marrow suppression (1 adult with neutropenia and thrombocytopenia after a single dose of 6000 mg), reversible neutropenia or granulocytopenia (4 adults, overdoses ranging from 8 mg/kg daily for 4 days to a single dose of 25 mg/kg), hepatitis (1 adult receiving 10 mg/kg daily, and one 2 kg infant after a single 40 mg dose), renal toxicity (1 adult with transient worsening of hematuria after a single 500 mg dose, and 1 adult with elevated creatinine (5.2 mg/dL) after a single 5000 to 7000 mg dose), and seizure (1 adult with known seizure disorder after 3 days of 9 mg/kg). In addition, 1 adult received 0.4 mL (instead of 0.1 mL) ganciclovir for injection solution by intravitreal injection, and experienced temporary loss of vision and central retinal artery occlusion secondary to increased intraocular pressure related to the injected fluid volume.
Since ganciclovir is dialyzable, dialysis may be useful in reducing serum concentrations. Adequate hydration should be maintained. The use of hematopoietic growth factors should be considered (see DOSAGE AND ADMINISTRATION , Renal Impairment).
-
DOSAGE AND ADMINISTRATION:
CAUTION – DO NOT ADMINISTER GANCICLOVIR FOR INJECTION, USP SOLUTION BY RAPID OR BOLUS INTRAVENOUS INJECTION. THE TOXICITY OF GANCICLOVIR FOR INJECTION, USP MAY BE INCREASED AS A RESULT OF EXCESSIVE PLASMA LEVELS.
CAUTION – INTRAMUSCULAR OR SUBCUTANEOUS INJECTION OF RECONSTITUTED GANCICLOVIR FOR INJECTION, USP SOLUTION MAY RESULT IN SEVERE TISSUE IRRITATION DUE TO HIGH pH (11).
Dosage
THE RECOMMENDED DOSE FOR GANCICLOVIR FOR INJECTION, USP SOLUTION SHOULD NOT BE EXCEEDED. THE RECOMMENDED INFUSION RATE FOR GANCICLOVIR FOR INJECTION SOLUTION SHOULD NOT BE EXCEEDED.
For Treatment of CMV Retinitis in Patients with Normal Renal Function
Induction Treatment
The recommended initial dosage for patients with normal renal function is 5 mg/kg (given intravenously at a constant rate over 1 hour) every 12 hours for 14 to 21 days.
Maintenance Treatment
Following induction treatment, the recommended maintenance dosage of ganciclovir for injection solution is 5 mg/kg given as a constant-rate intravenous infusion over 1 hour once daily, 7 days per week or 6 mg/kg once daily, 5 days per week.
For patients who experience progression of CMV retinitis while receiving maintenance treatment with either formulation of ganciclovir, reinduction treatment is recommended.
For the Prevention of CMV Disease in Transplant Recipients with Normal Renal Function
The recommended initial dosage of ganciclovir for injection solution for patients with normal renal function is 5 mg/kg (given intravenously at a constant rate over 1 hour) every 12 hours for 7 to 14 days, followed by 5 mg/kg once daily, 7 days per week or 6 mg/kg once daily, 5 days per week.
The duration of treatment with ganciclovir for injection solution and in transplant recipients is dependent upon the duration and degree of immunosuppression. In controlled clinical trials in bone marrow allograft recipients, treatment with ganciclovir for injection was continued until day 100 to 120 posttransplantation. CMV disease occurred in several patients who discontinued treatment with ganciclovir for injection solution prematurely. In heart allograft recipients, the onset of newly diagnosed CMV disease occurred after treatment with ganciclovir for injection was stopped at day 28 posttransplant, suggesting that continued dosing may be necessary to prevent late occurrence of CMV disease in this patient population (see INDICATIONS AND USAGE section for a more detailed discussion).
Renal Impairment
For patients with impairment of renal function, refer to Table 8 for recommended doses of ganciclovir solution and adjust the dosing interval as indicated:
Table 8 Dosing for Patients with Renal Impairment
Creatinine
Clearance*
(mL/min)
Ganciclovir for Injection
Induction
Dose (mg/kg)
Dosing
Interval
(hours)
Ganciclovir for Injection
Maintenance
Dose (mg/kg)
Dosing
Interval
(hours)
≥70
5
12
5
24
50 to 69
2.5
12
2.5
24
25 to 49
2.5
24
1.25
24
10 to 24
1.25
24
0.625
24
<10
1.25
3 times per week, following hemodialysis
0.625
3 times per week, following hemodialysis
*Creatinine clearance can be related to serum creatinine by the formulas given below.
Creatinine clearance for males = (140-age[yrs]) (body wt [kg])
(72) (serum creatinine [mg/dL])
Creatinine clearance for females = 0.85 x male value
Dosing for patients undergoing hemodialysis should not exceed 1.25 mg/kg 3 times per week, following each hemodialysis session. Ganciclovir for injection should be given shortly after completion of the hemodialysis session, since hemodialysis has been shown to reduce plasma levels by approximately 50%.
Patient Monitoring
Due to the frequency of granulocytopenia, anemia and thrombocytopenia in patients receiving ganciclovir (see ADVERSE EVENTS), it is recommended that complete blood counts and platelet counts be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in cytopenia, or in whom neutrophil counts are less than 1000 cells/mcL at the beginning of treatment. Patients should have serum creatinine or creatinine clearance values followed carefully to allow for dosage adjustments in renally impaired patients (see DOSAGE AND ADMINISTRATION).
Reduction of Dose
Dosage reductions in renally impaired patients are required for ganciclovir for injection (see Renal Impairment). Dosage reductions should also be considered for those with neutropenia, anemia and/or thrombocytopenia (see ADVERSE EVENTS ). Ganciclovir should not be administered in patients with severe neutropenia (ANC less than 500/mcL) or severe thrombocytopenia (platelets less than 25,000/mcL).
Method of Preparation of Ganciclovir for Injection Solution
Each 10 mL clear glass vial contains ganciclovir sodium equivalent to 500 mg of ganciclovir and 46 mg of sodium. The contents of the vial should be prepared for administration in the following manner:
1. Reconstituted Solution:
a. Reconstitute lyophilized ganciclovir for injection by injecting 10 mL of Sterile Water for Injection, USP into the vial.
DO NOT USE BACTERIOSTATIC WATER FOR INJECTION
CONTAINING PARABENS. IT IS INCOMPATIBLE WITH
GANCICLOVIR FOR INJECTION AND MAY CAUSE PRECIPITATION.
b. Shake the vial to dissolve the drug.
c. Visually inspect the reconstituted solution for particulate matter and discoloration prior to proceeding with infusion solution. Discard the
vial if particulate matter or discoloration is observed.
d. Reconstituted solution in the vial is stable at room temperature for 12 hours. It should not be refrigerated.
2. Infusion Solution:
Based on patient weight, the appropriate volume of the reconstituted solution (ganciclovir concentration 50 mg/mL) should be removed from the
vial and added to an acceptable infusion fluid (typically 100 mL) for delivery over the course of 1 hour. Infusion concentrations greater than
10 mg/mL are not recommended. The following infusion fluids have been determined to be chemically and physically compatible with
ganciclovir for injection solution: 0.9% Sodium Chloride, 5% Dextrose, Ringer’s Injection and Lactated Ringer’s Injection, USP.
Ganciclovir for injection, when reconstituted with sterile water for injection, further diluted with 0.9% sodium chloride injection, and stored
refrigerated at 5°C in polyvinyl chloride (PVC) bags, remains physically and chemically stable for 14 days.
However, because ganciclovir for injection is reconstituted with non-bacteriostatic sterile water, it is recommended that the infusion solution be
used within 24 hours of dilution to reduce the risk of bacterial contamination. The infusion should be refrigerated. Freezing is not
recommended.
Handling and Disposal
Caution should be exercised in the handling and preparation of solutions of ganciclovir for injection. Solutions of ganciclovir for injection are alkaline (pH 11). Avoid direct contact of the skin or mucous membranes with ganciclovir for injection solutions. If such contact occurs, wash thoroughly with soap and water; rinse eyes thoroughly with plain water.
Because ganciclovir shares some of the properties of anti-tumor agents (ie, carcinogenicity and mutagenicity), consideration should be given to handling and disposal according to guidelines issued for antineoplastic drugs. Several guidelines on this subject have been published.7-9
There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
- HOW SUPPLIED:
-
REFERENCES:
- Spector SA, Weingeis T, Pollard R, et al. A randomized, controlled study of intravenous ganciclovir therapy for cytomegalovirus peripheral retinitis in patients with AIDS. J Inf Dis. 1993; 168:557-563.
- Drew WL, Ives D, Lalezari JP, et al. Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. New Engl J Med. 1995; 333:615-620.
- The Oral Ganciclovir European and Australian Cooperative Study Group. Intravenous vs oral ganciclovir: European/Australian comparative study of efficacy and safety in the prevention of cytomegalovirus retinitis recurrence in patients with AIDS. AIDS. 1995; 9:471-477.
- Merigan TC, Renlund DG, Keay S, et al. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. New Engl J Med. 1992; 326:1182-1186.
- Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. New Engl J Med. 1991; 325:1601-1607.
- Schmidt GM, Horak DA, Niland JC, et al. The City of Hope-Stanford-Syntex CMV Study Group. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants. New Engl J Med. 1991; 15:1005-1011.
- Recommendations for the Safe Handling of Cytotoxic Drugs. US Department of Health and Human Services, National Institutes of Health, Bethesda, MD, September 1992. NIH Publication No. 92-2621.
- American Society of Hospital Pharmacists technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990; 47:1033-1049.
- Controlling Occupational Exposures to Hazardous Drugs. US Department of Labor. Occupational Health and Safety Administration. OSHA Technical Manual. Section V - Chapter 3, September 22, 1995.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GANCICLOVIR
ganciclovir sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-315 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GANCICLOVIR SODIUM (UNII: 02L083W284) (GANCICLOVIR - UNII:P9G3CKZ4P5) GANCICLOVIR 500 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-315-10 25 in 1 TRAY 06/28/2010 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090658 06/28/2010 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 840771732 MANUFACTURE(63323-315)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

