TAUVID- flortaucipir f-18 injection, solution
TAUVID by
Drug Labeling and Warnings
TAUVID by is a Prescription medication manufactured, distributed, or labeled by Eli Lilly and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TAUVID safely and effectively. See full prescribing information for TAUVID.
TAUVID™ (flortaucipir F 18 injection), for intravenous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
TAUVID is a radioactive diagnostic agent indicated for positron emission tomography (PET) imaging of the brain to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease (AD). (1)
Limitations of Use
TAUVID is not indicated for use in the evaluation of patients for chronic traumatic encephalopathy (CTE). (1, 5.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 300 MBq/mL to 3,700 MBq/mL (8.1 mCi/mL to 100 mCi/mL) of flortaucipir F 18 in a multiple-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Risk of Misdiagnosis in Patients Evaluated for Alzheimer's disease: Consider additional evaluation to confirm the absence of AD pathology in patients with a negative TAUVID scan. (2.6, 5.1)
- Risk of Chronic Traumatic Encephalopathy Misdiagnosis: The safety and effectiveness of TAUVID have not been established for patients being evaluated for CTE. (1, 5.2)
- Radiation Risk: Ensure safe drug handling to protect patients and health care workers from unintentional radiation exposure. (2.1, 5.3)
ADVERSE REACTIONS
Most common adverse reactions (frequency ≥ 0.5%) were headache, injection site pain, and increased blood pressure. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Interrupt breastfeeding. Advise a lactating woman to avoid breastfeeding for 4 hours after TAUVID administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Recommended Dosage and Administration Instructions
2.3 Image Acquisition
2.4 Image Display
2.5 Preparation for Image Interpretation
2.6 Image Interpretation
2.7 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Misdiagnosis in Patients Evaluated for Alzheimer's disease
5.2 Risk of Chronic Traumatic Encephalopathy Misdiagnosis
5.3 Radiation Risk
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
TAUVID is indicated for use with positron emission tomography (PET) imaging of the brain to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease (AD).
Limitations of Use
TAUVID is not indicated for use in the evaluation of patients for chronic traumatic encephalopathy (CTE) [see Warnings and Precautions (5.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
TAUVID is a radioactive drug. Only authorized persons qualified by training and experience should receive, use, and administer TAUVID. Handle TAUVID with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.3)]. Use waterproof gloves and effective shielding, including syringe shields, when preparing and handling TAUVID.
2.2 Recommended Dosage and Administration Instructions
Recommended Dose
The recommended amount of radioactivity to be administered for PET imaging is 370 MBq (10 mCi), administered as an intravenous bolus injection in a total volume of 10 mL or less.
Preparation and Administration
- Assessment of pregnancy status is recommended in females of reproductive potential before administering TAUVID.
- Use aseptic technique and radiation shielding during the preparation and administration of TAUVID [see Dosage and Administration (2.1)].
- Visually inspect the radiopharmaceutical solution prior to administration. Do not use it if it contains particulate matter or if it is discolored (TAUVID is a clear, colorless solution).
- TAUVID may be diluted aseptically with 0.9% Sodium Chloride Injection to a maximum dilution of 1:5 by the end-user. Diluted product should be used within 4 hours of dilution and prior to product expiry.
- Assay the dose in a suitable dose calibrator prior to administration.
2.3 Image Acquisition
Starting approximately 80 minutes after the TAUVID intravenous injection, obtain a 20-minute PET image with the patient supine. Position the head to center the brain (including the cerebellum) in the PET scanner field of view. Tape or other flexible head restraints may be used to reduce head movement.
2.4 Image Display
The goal of the read is to identify and locate areas of flortaucipir activity in the neocortex that are greater than the background activity (background activity is defined as up to 1.65-fold the measured cerebellar average). For optimal display, select a color scale with a rapid transition between two distinct colors and adjust the scale so that the transition occurs at the 1.65-fold threshold. Examine the posterolateral temporal (PLT), occipital, parietal, and frontal regions bilaterally. Neocortical activity in either hemisphere contributes to image interpretation. Activity in white matter or regions outside the brain does not contribute to image interpretation. To help identify the PLT, consider subdividing the temporal lobe into four quadrants as instructed below. Activity in the anterior and medial temporal lobe does not contribute to image interpretation of a positive TAUVID pattern.
Image Display and Orientation
Display images in the transverse, sagittal, and coronal planes. Reorient images to remove head tilt in the transverse and coronal plane. Use a sagittal slice just off the midline to align the inferior frontal and inferior occipital poles in the horizontal plane.
Select and Adjust the Color Scale
To create a visual threshold for positivity:
- Draw a region of interest around the cerebellum in the transverse plane.
- Select the plane to go through the cerebellum at the maximum cross-sectional area of the cerebellum.
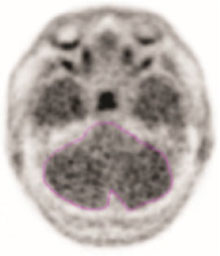
- Record the mean activity or cerebellar counts (MCC). The region of interest should be drawn with the scan in gray scale and in the transverse plane as seen in the example in Figure 1.
Figure 1: Example of Cerebellar Region of Interest
- Select a color scale for image display that has a rapid transition between two distinct colors in the general range of 25% to 60% of maximum intensity.
- Set the upper contrast value (UCV) of the color scale. Use the following formula to set the visual threshold of 1.65 x MCC to match the rapid transition in the color scale:
UCV = (MCC x 1.65) x (100% / % level of color transition) If additional guidance on image display is needed, refer to the TAUVID User Guide for PET Image Display available by request from the manufacturer.
2.5 Preparation for Image Interpretation
- Before interpreting the image, review the brain to determine the lobar anatomy. Interpret the images by first evaluating the temporal lobes, followed by occipital, parietal, and frontal lobes bilaterally.
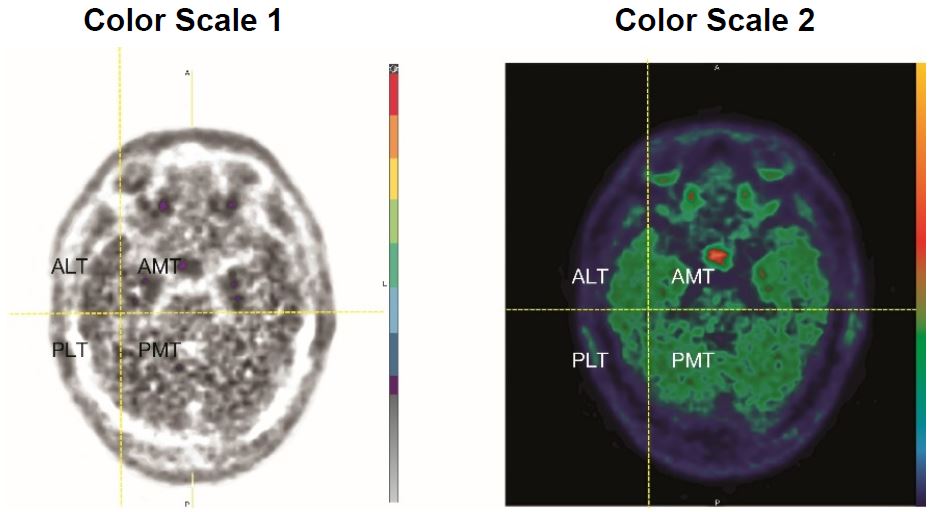
- To evaluate the temporal lobes, subdivide them into four quadrants by placing the horizontal crosshair immediately posterior to the brainstem nuclei and then scrolling inferiorly to place the vertical crosshair through the widest portion of the temporal pole, thus obtaining the anterolateral temporal (ALT), anterior mesial temporal (AMT), posterolateral temporal (PLT) and posterior mesial temporal (PMT) quadrants. See Figure 2 for an example (the left and right image panels show the same scan in two different color scales).
2.6 Image Interpretation
Interpret TAUVID imaging independently of the patient's clinical features and other imaging.
Interpret the PET TAUVID images based upon the pattern and density of the radioactive signal within the neocortical gray matter (not within white matter or in regions outside of the brain). Only uptake of tracer in the neocortical grey matter regions should contribute to scan interpretation.
Off-target binding may be seen in the choroid plexus, striatum, and brainstem nuclei. Small foci of noncontiguous tracer uptake may lead to false positive interpretation. Interpret scans that have isolated or noncontiguous, small foci in any region with caution. Some scans may be difficult to interpret due to image noise or motion artifact. For cases where there is uncertainty as to the location of neocortical uptake, use co-registered anatomical imaging to improve localization of uptake.
Positive TAUVID Scan
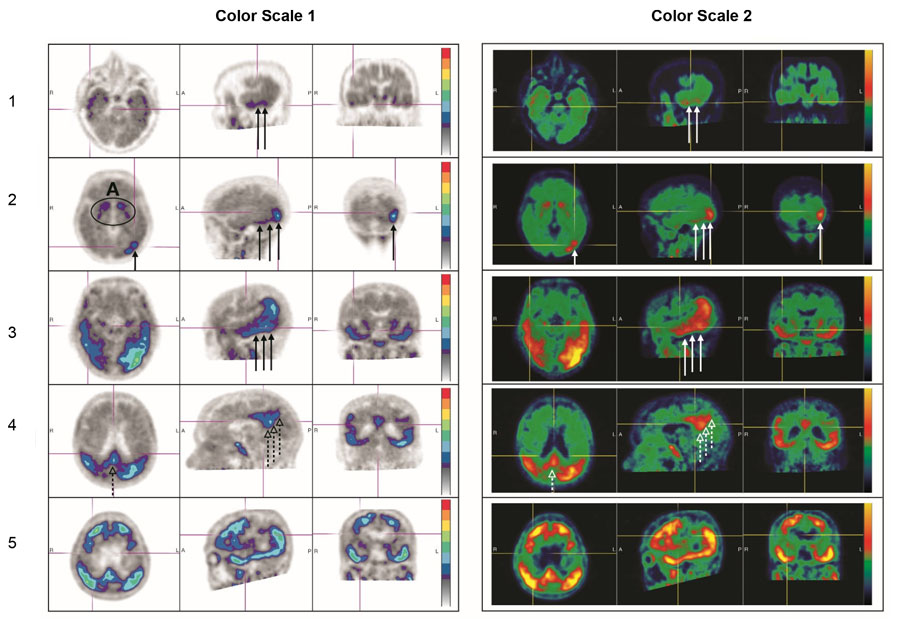
A positive scan shows increased neocortical activity in posterolateral temporal (PLT), occipital, or parietal/precuneus region(s), with or without frontal activity. Neocortical activity in either hemisphere can contribute to identification of the positive pattern. A positive scan supports the presence of widely distributed tau neuropathology (B3 tau pathology). See Figure 3 for examples (the left and right image panels show the same scans in two different color scales) [see Warnings and Precautions (5.1)].
Negative TAUVID Scan
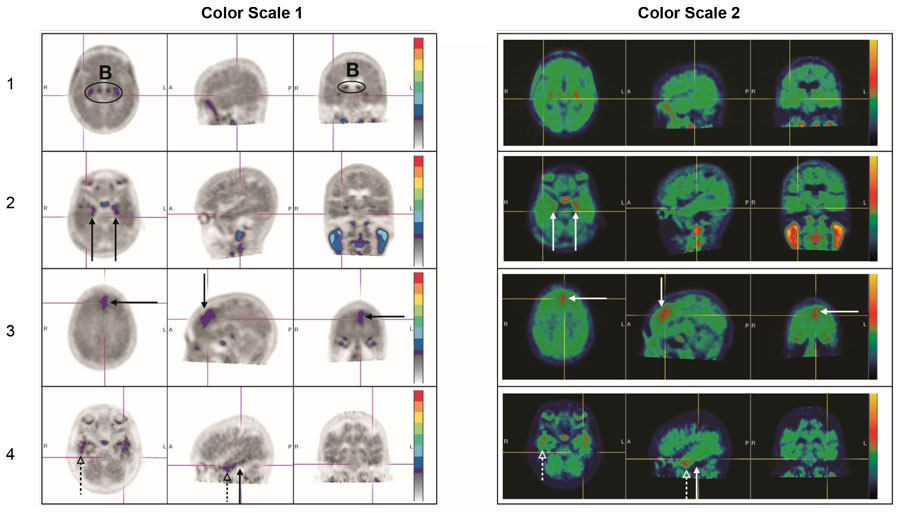
A negative scan shows no increased neocortical activity, or shows increased neocortical activity isolated to the mesial temporal, anterolateral temporal, and/or frontal regions. See Figure 4 for examples (the left and right image panels show the same scans in two different color scales) [see Warnings and Precautions (5.1)].
Figure 3: Positive Scan Examples
A: Off target binding in the striatum.
Row 1: Example of a patient with increased uptake in PLT.
Row 2: Example of a patient with increased uptake in PLT and occipital regions.
Rows 3 and 4: Example of a patient with increased neocortical activity in PLT, occipital lobe (solid arrows) and precuneus (dashed arrows) (row 3: level of temporal lobes, row 4: level of parietal/precuneus).
Row 5: Example of a patient with increased neocortical activity in medial prefrontal/cingulate, lateral prefrontal, PLT, parietal, occipital and precuneus regions.
Figure 4: Negative Scan Examples
B: Off target binding in the choroid plexus or brainstem nuclei.
Row 1: Example of a patient with no increased neocortical activity (activity is similar in intensity to cerebellar reference region).
Row 2: Example of a patient with increased activity isolated to MTL.
Row 3: Example of a patient with increased neocortical activity isolated to frontal lobe.
Row 4: Example of a patient with small isolated foci of non-contiguous and variable uptake in the PLT (solid arrows); increased activity in the ALT (dashed arrows). This pattern may also be seen in the occipital or parietal region.
2.7 Radiation Dosimetry
Radiation absorbed dose estimates are shown in Table 1 for organs and tissues of adults from intravenous administration of TAUVID. The effective radiation dose resulting from administration of 370 MBq (10 mCi) of TAUVID to an adult weighing 70 kg is estimated to be 8.7 mSv. Critical organs include the upper large intestinal wall, small intestine, and liver. When PET/CT is performed, exposure to radiation will increase in an amount dependent on the settings used in the CT acquisition.
Table 1: Estimated Radiation Absorbed Dose After a TAUVID Injection a Assumed radiation weighting factor, wr, (formerly defined as quality factor, Q) of 1 for conversion of absorbed dose (Gray or rads) to dose equivalent (Sieverts or rem) for F 18. To obtain radiation absorbed dose in rad/mCi from above table, multiply the dose in μGy/MBq by 0.0037 (e.g., 14 μGy/MBq x 0.0037 = 0.0518 rad/mCi).
Organ/Tissue Mean Absorbed Dose Per Unit
Administered Activity (μGy/MBq)Adrenal glands 14 Brain 8 Breasts 7 Gallbladder wall 38 Lower large intestine wall 35 Small intestine wall 85 Stomach wall 13 Upper large intestine wall 96 Heart wall 30 Kidneys 40 Liver 57 Lungs 34 Muscle 9 Ovaries 21 Pancreas 14 Red bone marrow 10 Osteogenic cells 12 Skin 6 Spleen 10 Testes 7 Thymus gland 9 Thyroid 7 Urinary bladder wall 38 Uterus 18 Total body 12 Effective dose (μSv/MBq)a 24 - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Misdiagnosis in Patients Evaluated for Alzheimer's disease
TAUVID does not target β-amyloid, one of two required components of the neuropathological diagnosis of AD.
TAUVID performance for detecting tau pathology was assessed in terminally ill patients, the majority of whom had AD dementia with B3 level NFT pathology. TAUVID performance for detecting tau pathology may be lower in patients in earlier stages of the pathological spectrum [see Clinical Studies (14)].
Negative TAUVID Scan
NFTs may be present at levels that qualify for the neuropathological diagnosis of AD (B2 tau pathology in the presence of at least moderate levels of cortical amyloid pathology) in patients with a negative TAUVID scan. Consider additional evaluation to confirm the absence of AD pathology in patients with a negative TAUVID scan.
False Positive TAUVID Scan
Small foci of noncontiguous tracer uptake may lead to a false positive TAUVID scan. Only uptake of tracer in the neocortex should contribute to the interpretation of a positive TAUVID scan [see Dosage and Administration (2.4)].
5.2 Risk of Chronic Traumatic Encephalopathy Misdiagnosis
The safety and effectiveness of TAUVID have not been established for patients being evaluated for CTE. Preliminary non-clinical and clinical investigations suggest differences in tau conformation and distribution may limit flortaucipir F 18 binding. Therefore, TAUVID is not indicated for detection of CTE.
5.3 Radiation Risk
Diagnostic radiopharmaceuticals, including TAUVID, expose patients to radiation [see Dosage and Administration (2.7)]. Radiation exposure is associated with a dose-dependent increased risk of cancer. Ensure safe handling and preparation procedures to protect patients and health care workers from unintentional radiation exposure [see Dosage and Administration (2.1, 2.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies, 1,921 study participants were exposed to TAUVID [see Clinical Studies (14)]. In these studies, 885 study participants received 240 MBq of TAUVID (about 65% of the recommended dose) and 1,036 study participants received 370 MBq of TAUVID (the recommended dose). The adverse reactions reported in ≥ 0.5% of study participants are shown in Table 2.
Table 2: Adverse Reactions with a Frequency ≥0.5% in Adults Who Received TAUVID in Clinical Trials (n = 1,921) Adverse Reaction n (%) Headache 26 (1.4%) Injection site pain 23 (1.2%) Increased blood pressure 15 (0.8%) Adverse reactions with a frequency <0.5% in adults who received TAUVID in clinical trials include:
Nervous system disorders: dysgeusia
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All radiopharmaceuticals, including TAUVID, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with administration of TAUVID. TAUVID is not likely to be used in females of reproductive age.
There are no available data on TAUVID use in pregnant women. No animal reproduction studies using flortaucipir F 18 have been conducted to evaluate its effect on female reproduction and embryo-fetal development.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of flortaucipir F 18 in human milk, or its effects on the breastfed infant or milk production. Lactation studies have not been conducted in animals. Advise a lactating woman to avoid breastfeeding for 4 hours after TAUVID administration in order to minimize radiation exposure to a breastfed infant.
8.4 Pediatric Use
The safety and effectiveness of TAUVID in pediatric patients have not been established.
8.5 Geriatric Use
Of 1,921 study participants in completed clinical studies of TAUVID, 1,544 (80%) TAUVID-treated subjects were ≥ 65 years old, while 839 (44%) were ≥ 75 years old. No overall differences in safety or effectiveness of TAUVID were observed between subjects ≥ 65 years old and younger adult subjects, and other reported clinical experience has not identified differences in safety or effectiveness between elderly and younger patients.
-
11 DESCRIPTION
11.1 Chemical Characteristics
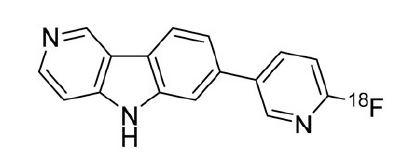
TAUVID contains flortaucipir fluorine 18 (F 18). Chemically, flortaucipir F 18 is 7-(6-[F-18]fluoropyridin-3-yl)-5H-pyrido[4,3-b]indole. The molecular weight is 262.27, the molecular formula is C16H10[18F]N3, and the structural formula is:
TAUVID is a sterile, non-pyrogenic solution for intravenous injection. The clear, colorless solution free of visible particulate matter is supplied ready to use, and each milliliter contains up to 2 micrograms of flortaucipir and 300 to 3,700 MBq (8.1 to 100 mCi) flortaucipir F 18 at end of synthesis, 1.5 mg of L-cysteine hydrochloride monohydrate USP, 2.1 mg of dibasic sodium phosphate anhydrous USP, up to 55 of micrograms hydrogen chloride and 0.1 mL dehydrated alcohol in 0.9% sodium chloride injection USP. The pH of the solution is between 5.5 and 7.5.
11.2 Physical Characteristics
TAUVID is radiolabeled with fluorine 18 (F 18), a cyclotron produced radionuclide that decays by positron emission to stable oxygen 18 with a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 3).
Table 3: Principal Radiation Produced from Decay of Fluorine 18 Radiation Energy Level (keV) Abundance (%) Positron 249.8 96.9 Gamma 511 193.5 11.3 External Radiation
The point source air-kerma coefficient for F18 is 3.74E-17 Gy m2/(Bq s). The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 4. The use of 8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 4: Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding Shield Thickness
cm of lead (Pb)Coefficient of Attenuation 0.6 0.5 2 0.1 4 0.01 6 0.001 8 0.0001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flortaucipir F 18 binds to aggregated tau protein. In the brains of patients with AD, tau aggregates combine to form NFTs, one of two components required for the neuropathological diagnosis of AD. In vitro, flortaucipir F 18 binds to paired helical filament (PHF) tau purified from brain homogenates of donors with AD. The dissociation constant (Kd) of flortaucipir F 18 binding to PHFs is 0.57 nM. In vivo, flortaucipir F 18 is differentially retained in neocortical areas that contain aggregated tau. In vitro, tritiated flortaucipir has been reported to bind with low nanomolar affinity to monoamine oxidase-A and monoamine oxidase-B, which could contribute to off target binding.
12.2 Pharmacodynamics
The relationship between flortaucipir F 18 plasma concentrations and image interpretation was not explored in clinical trials.
Effect of MAO Inhibitors on Flortaucipir Binding in AD Patients
TAUVID PET signal was slightly reduced by rasagiline, a MAO-B inhibitor, in vivo in low tau, high MAO-B areas of the brain such as the nucleus accumbens, putamen, and caudate. However, there is little potential for MAO binding to affect TAUVID scan interpretation in neocortical areas.
12.3 Pharmacokinetics
After intravenous administration of TAUVID, flortaucipir F 18 was distributed throughout the body with less than 10% of the injected F 18 radioactivity present in the blood by 5 minutes following administration, and less than 5% present in the blood by 10 minutes after administration. The residual F 18 in circulation during the 80-minute to 100-minute imaging window was approximately 28% to 34% parent, with the remainder being metabolites.
Clearance occurs primarily by hepatobiliary and renal excretion.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies to assess the carcinogenicity or reproductive toxicity potentials of flortaucipir F 18 have not been conducted.
In an in vitro bacterial reverse mutation assay (Ames test), increases in the number of revertant colonies were observed in 4 of the 5 strains exposed to flortaucipir F 19. In a chromosomal aberration in vitro study with Chinese hamster ovary (CHO) cells, flortaucipir F 19 increased the percent of cells with structural aberrations with 3 hour exposure with or without S9 metabolic activation. Twenty hour exposure without activation produced an increase in structural aberrations at all tested concentrations.
Flortaucipir F 19 was evaluated in a rat micronucleus study and showed no genotoxicity. In this study, flortaucipir F 19 did not increase the number of micronucleated polychromatic erythrocytes at the highest achievable dose level, 1600 μg/kg/day, when given for two consecutive days.
-
14 CLINICAL STUDIES
The performance of TAUVID imaging to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) was evaluated in two clinical studies: Study 1 (NCT02516046) and Study 2 (NCT03901092). In each study, TAUVID imaging was interpreted by 5 independent readers who were blinded to clinical information. Readers interpreted TAUVID imaging as positive or negative [see Dosage and Administration (2.6)].
Study 1 enrolled 156 terminally ill patients who agreed to undergo TAUVID imaging and to participate in a postmortem brain donation program. In 64 of these patients, reader interpretation of the TAUVID scan was compared to tau pathology based on scoring provided by independent pathologists, who evaluated the density and distribution of NFTs in the post-mortem brain (see Table 5). Of the 64 patients, the mean age was 83 years (range 55 to 100); 34 were female; 49 had dementia, 1 had mild cognitive impairment, and 14 had no cognitive impairment on clinical evaluation around the time of TAUVID imaging.
Table 5: Study 1 Tau Pathology Scoring Tau Pathology Score Distribution of Tau NFTs in the Brain B0 No NFTs B1 NFTs limited to transentorhinal brain region B2 B1 + NFTs limited to limbic brain regions B3 B2 + NFTs distributed throughout the neocortex Image reader performance for distinguishing B3 (positive) from B0-B2 (negative) tau pathology is shown in Table 6.
Table 6: Study 1 TAUVID Scan Reader Performance for B3 Tau Pathology a CI = confidence interval.
Reader True Positive True Negative False Positive False Negative Sensitivity % (95% CIa) Specificity % (95% CI) 1 38 17 8 1 97
(87, 100)68
(48, 83)2 36 23 2 3 92
(80, 97)92
(75, 98)3 36 22 3 3 92
(80, 97)88
(70, 96)4 36 19 6 3 92
(80, 97)76
(57, 89)5 39 13 12 0 100
(91, 100)52
(34, 70)The performance of the five TAUVID readers for sensitivity (95% CI) ranged from 92% (80, 97) to 100% (91, 100) and for specificity (95% CI) ranged from 52% (34, 70) to 92% (75, 98). Exploratory analysis evaluated how the same TAUVID interpretations distinguished B2-B3 from B0-B1 tau pathology, a threshold used in integrating tau and amyloid pathology for the neuropathological diagnosis of AD. In this analysis, the performance of the five TAUVID readers for sensitivity (95% CI) ranged from 68% (55, 79) to 86% (74, 93) and for specificity (95% CI) ranged from 63% (31, 86) to 100% (68, 100) [see Warnings and Precautions (5.1)].
Study 2 included the same terminally ill patients as in Study 1 (plus 18 additional terminally ill patients) and 159 patients with cognitive impairment being evaluated for AD (the indicated population). Inter-reader agreement for five new TAUVID readers was evaluated using Fleiss' kappa statistic (95% CI) and found to be 0.87 (0.83, 0.91) across all 241 patients. Exploratory analysis evaluated inter-reader agreement in two subgroups. In this analysis, Fleiss' kappa (95% CI) was 0.82 (0.75, 0.88) in the terminally ill patients and 0.90 (0.85, 0.95) in the indicated population.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TAUVID injection is supplied in a 50 mL or 100 mL multiple-dose vial containing a clear, colorless solution free of visible particulate matter at a strength of 300 MBq/mL to 3,700 MBq/mL (8.1 mCi/mL to 100 mCi/mL) flortaucipir F 18 at end of synthesis. Each vial contains multiple doses and is enclosed in a shield container to minimize external radiation exposure.
50 mL NDC: 0002-1220-50 (IC1220) 100 mL NDC: 0002-1220-48 (IC1220) 16.2 Storage and Handling
Storage
Store TAUVID at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. TAUVID does not contain a preservative. Store TAUVID upright in a shielding container [see Dosage and Administration (2.1)]. The expiration date and time are provided on the container label. Use TAUVID within the labeled expiration.
-
17 PATIENT COUNSELING INFORMATION
Radiation Risk
Advise patients of the radiation risk of TAUVID [see Warnings and Precautions (5.3)].
Pregnancy
Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with TAUVID [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to avoid breastfeeding for 4 hours after TAUVID administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
Manufactured for Avid Radiopharmaceuticals, a wholly-owned subsidiary of Eli Lilly and Company, Philadelphia, PA 19104
Copyright © 2020, 2024, Eli Lilly and Company. All rights reserved.
TAU-0003-USPI-20240205
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Tauvid 100 mci 50 mL SHIELD
NDC: 0002-1220-50
Tauvid™
Flortaucipir F 18 Injection
50 mL Multiple-Dose Vial
Sterile
Rx Only
CAUTION ☢ RADIOACTIVE MATERIAL
300 MBq/mL to 3,700 MBq/mL (8.1 mCi/mL to 100 mCi/mL) at End of Synthesis
For Intravenous Use Only
_____MBq (_____mCi) in _____mL at _____:_____ on _____
Expires at _____:_____ on _____
Batch No._____
Dosage: See prescribing information.
Contains up to 2 micrograms of flortaucipir, 1.5 mg L-cysteine hydrochloride monohydrate USP, 2.1 mg of dibasic sodium phosphate anhydrous USP, up to 55 micrograms of hydrogen chloride and 0.1 mL dehydrated alcohol USP in 0.9% sodium chloride injection USP per milliliter of solution. Store vial upright in a shielding container at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Manufactured by PETNET Solutions, Inc. Knoxville, TN 37932 for Avid Radiopharmaceuticals, a wholly-owned subsidiary of Eli Lilly and Company, Philadelphia, PA 19104
-
INGREDIENTS AND APPEARANCE
TAUVID
flortaucipir f-18 injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-1220 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Flortaucipir F-18 (UNII: T1JP1KYU9O) (Flortaucipir F-18 - UNII:T1JP1KYU9O) Flortaucipir F-18 100 mCi in 1 mL Inactive Ingredients Ingredient Name Strength Cysteine Hydrochloride (UNII: ZT934N0X4W) 1.5 mg in 1 mL Sodium phosphate, Dibasic, Anhydrous (UNII: 22ADO53M6F) 2.1 mg in 1 mL Alcohol (UNII: 3K9958V90M) 78.9 mg in 1 mL Sodium Chloride (UNII: 451W47IQ8X) 8.1 mg in 1 mL Hydrochloric Acid (UNII: QTT17582CB) 55 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-1220-50 1 in 1 CAN 10/25/2022 1 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC: 0002-1220-48 1 in 1 CAN 10/25/2022 2 100 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212123 07/01/2022 Labeler - Eli Lilly and Company (006421325) Registrant - Eli Lilly and Company (006421325)
Trademark Results [TAUVID]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TAUVID 98240193 not registered Live/Pending |
Eli Lilly and Company 2023-10-25 |
 TAUVID 97017182 not registered Live/Pending |
Avid Radiopharmaceuticals, Inc. 2021-09-08 |
 TAUVID 87866048 not registered Live/Pending |
Eli Lilly and Company 2018-04-06 |
 TAUVID 86412141 not registered Dead/Abandoned |
Avid Radiopharmaceuticals, Inc. 2014-10-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.