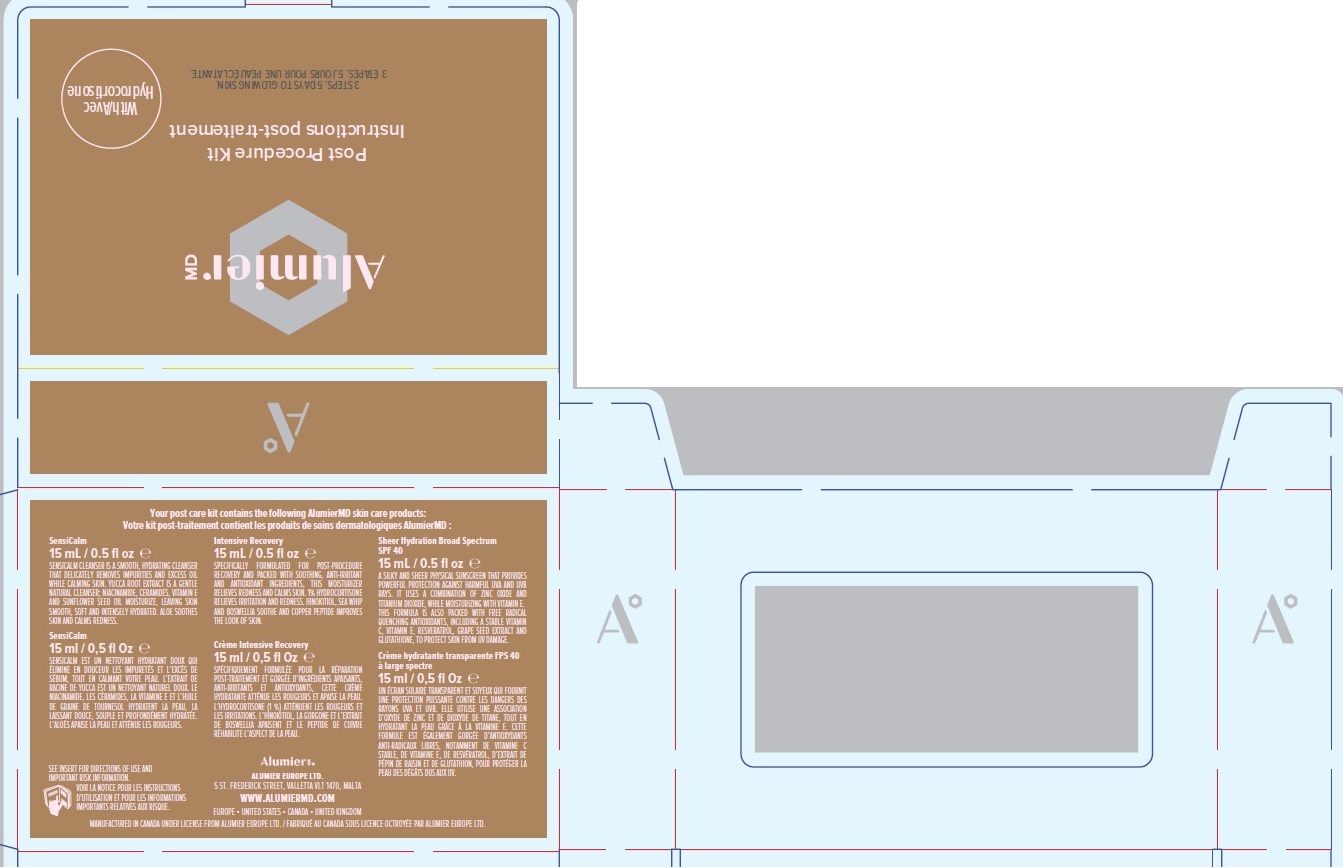
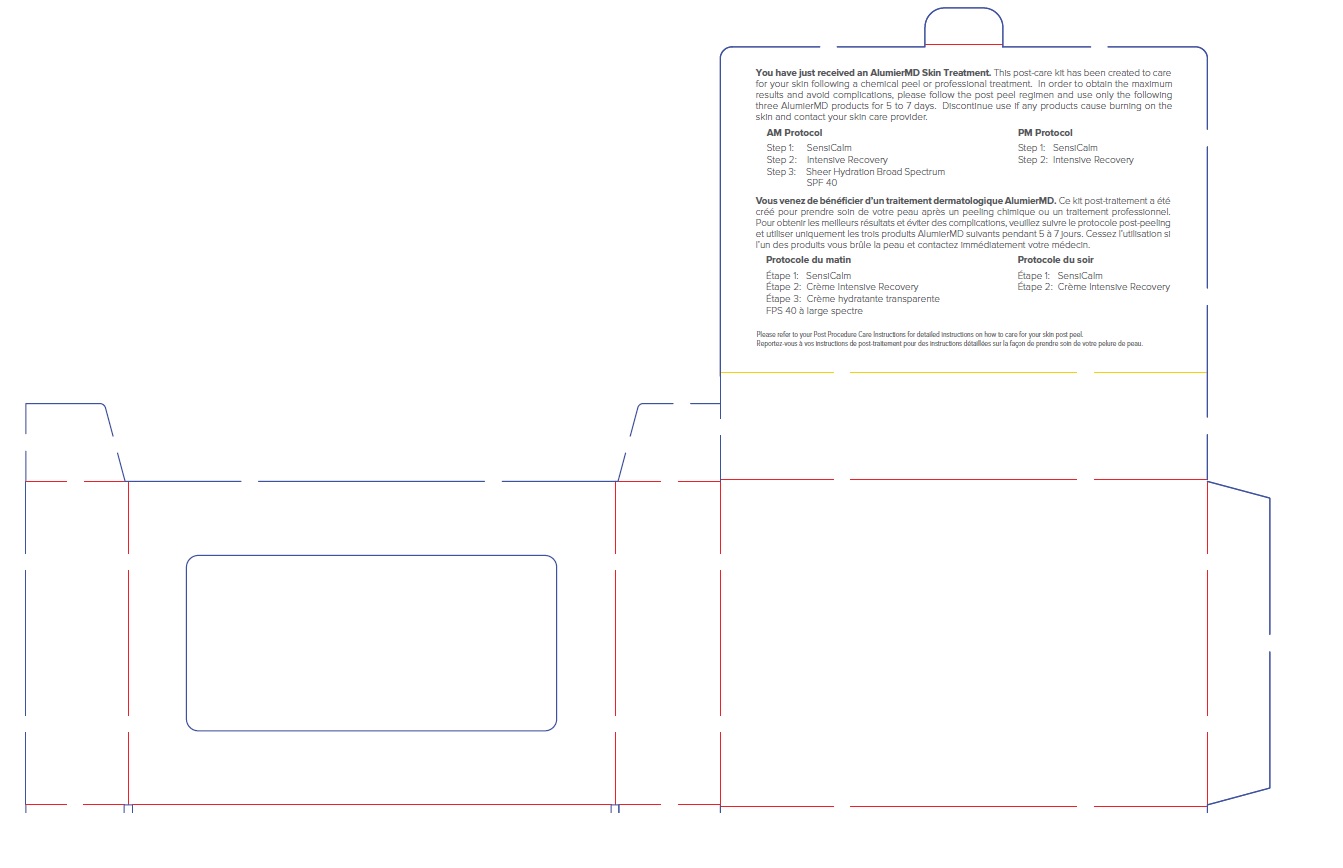
ALUMIER MD POST PROCEDURE KIT WITH HYDROCORTISONE- titanium dioxide, zinc oxide, hydrocortisone acetate
Alumier Labs
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts - Intensive Recovery
Active Ingredient
Hydrocortisone (Hydrocortisone acetate) 1.0%
Uses
- adults and children 2 years of age and older: For the temporary relief of minor skin irritations, rashes, itching and redness due to eczema, insect bites, poison ivy, poison oak, poison sumac, contact dermatitis (e.g. caused by soaps, detergents, cosmetics and/or jewelry), seborrheic dermatitis, psoriasis.
Warnings
-
do not use for the treatment of diaper rash, except on the advise of a physician
When using this product
- avoid contact with eyes, do not use in or around the eyes
Stop use and ask a doctor if condition worsens or if symptoms persist for more than 7 days or clears up and occurs again within a few days. Do not begin use of any other hydrocortisone product unless directed by a physician.
Keep out of reach of children. If swallowed, call a Poison Control Centre or get medical help right away.
Directions
- adults and children 2 years of age and older, apply sparingly to affected area not more than three to four times daily
-
do not use on children under 2 years of age, consult a physician
Other Information
- Do not use if security seal is broken or missing.
- You may report a serious adverse reaction event from using this product to: Alumier Labs Inc., 550 Cochituate Road, Suite 25, Framingham, MA 01701
Inactive Ingredients
Water/Aqua/Eau, Butyrospermum Parkii (Shea Butter), Simmondsia Chinensis (Jojoba) Seed Oil, Glycerin, Dimethicone, Caprylic/Capric Triglyceride, Behenyl Alcohol, Theobroma Cacao (Cocoa) Seed Butter, Sodium Acrylates Copolymer, Niacinamide, Aloe Barbadensis Leaf Juice, Hinokitiol, Resveratrol, Lavandula Angustifolia (Lavender) Flower/Leaf/Stem Extract, Anthemis Nobilis Flower Extract, Sea Whip Extract, Boswellia Serrata Extract, Honey Extract (Extrait de Miel), Tetrapeptide-14, Copper Tripeptide-1, Camellia Sinensis Leaf Extract, Aspalathus Linearis (Rooibos) Extract, Eucalyptus Globulus Leaf Extract, Mentha Viridis (Spearmint) Extract, Pogostemon Cablin Leaf Extract, Pyrus Malus (Apple) Fruit Extract, Rose Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Vaccinium Macrocarpon (Cranberry) Fruit Extract, Hydrogenated Lecithin, Squalane, Lecithin, Xanthan Gum, Caprylic Acid, Pentylene Glycol, Isosqualane, Sodium Methyl Stearoyl Taurate, Tricaprylin, Polymethyl Methacrylate, Sodium Hydroxide, Disodium EDTA, Caprylyl Glycol, Xylitol, Ethylhexylglycerin.
Drug Facts - Sheer Hydration
Active Ingredients
Titanium Dioxide 7%
Zinc Oxide 6.28%
Warnings
When using this product
- do not use on damaged or broken skin
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if you develop severe irritation, burning or itching of the skin, hives, swelling of eyes and mouth, blistering or difficulty breathing
Keep out of reach of children. If swallowed, call a Poison Control Centre or get medical help right away.
Directions
- apply liberally/generously (and evenly) 15 minutes before sun exposure
- reapply at least every 2 hours
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: - limit time in the sun, especially from 10 a.m. - 2 p.m. - wear long-sleeved shirts, pants, hats and sunglasses
- use a water resistant sunscreen if swimming or sweating
Other Information
- Do not use if security seal is broken or missing.
- You may report a serious adverse reaction event from using this product to: Alumier Labs Inc., Cochituate Road, Suite 25, Framingham, MA 01701
Inactive Ingredients
Water/Aqua/Eau, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Propanediol, Butyloctyl Salicylate, Cetearyl Alcohol, Aluminium Stearate, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Lecithin (Syn. Phosphatidycholine), Vitis Vinifera Seed Extract, Resveratrol, Glutathione, Tocopheryl Acetate, Magnesium Ascorbyl Phosphate, Acacia Senegal Gum, Synthetic Wax, Polyhydroxysteric Acid, Ceteareth-20, Alumina, Microcrystalline Cellulose, Triethoxycaprylylsilane, Xanthan Gum, Cellulose Gum, C20-4O Pareth-10, Hydroxyethyl Behenamidopropyl Dimonium Chloride, Polyquatemium-67, Disodium EDTA, Ethylhexylglycerin, Phenoxyethanol.
Company Information
Alumier MD
ALUMIER EUROPE LTD.
5 ST. FREDERICK STREET
VALLETTA VLT 1470, MALTA
WWW.ALUMIERMD.COM
EUROPE UNITED STATES CANADA UNITED KINGDOM
MANUFACTURED IN CANADA UNDER LICENSE FROM ALUMIER EUROPE LTD.