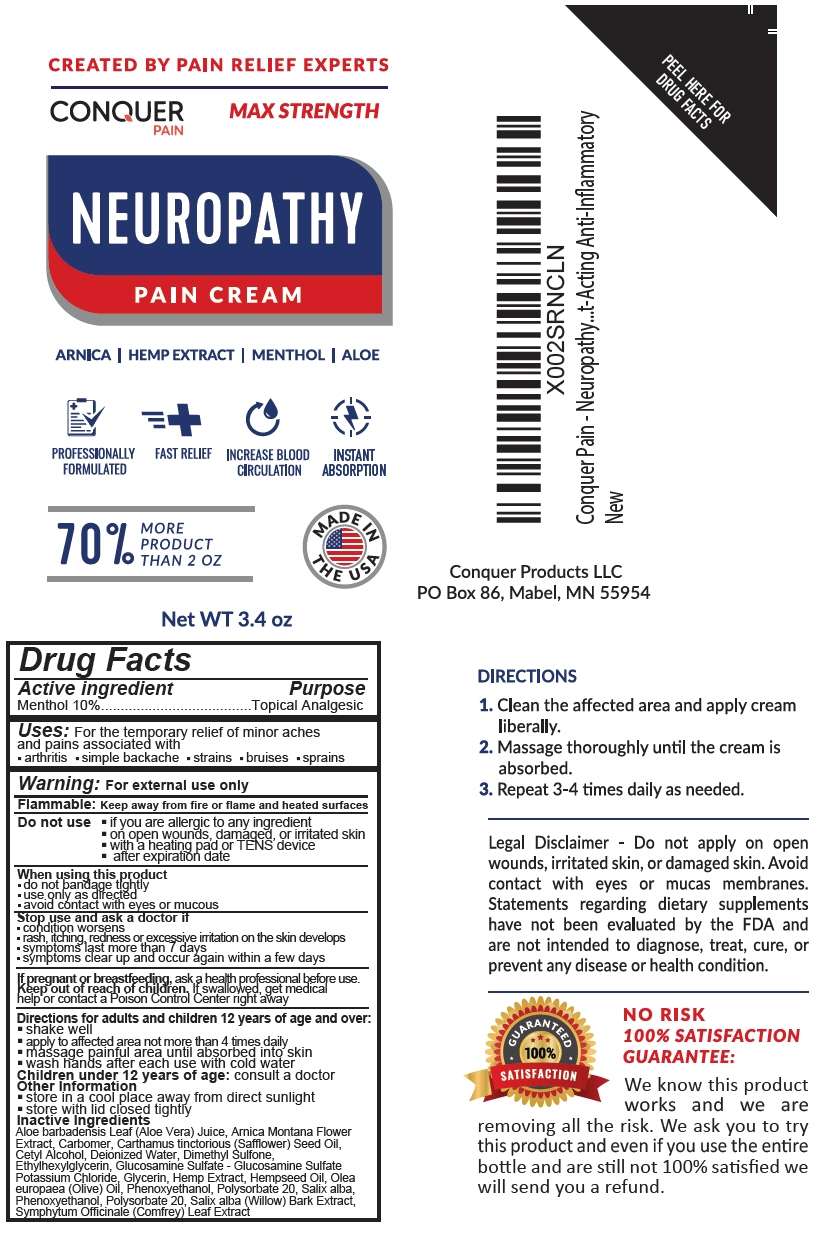
Conquer Pain Relief Cream
Conquer Pain Relief Cream by
Drug Labeling and Warnings
Conquer Pain Relief Cream by is a Otc medication manufactured, distributed, or labeled by Steuart Contract Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CONQUER PAIN RELIEF CREAM- menthol, unspecified form lotion
Steuart Contract Packaging
----------
Conquer Pain Relief Cream
Uses
For the temporary relief of minor aches and pains associated with
- arthritis
- simple backache
- strains
- bruises
- sprains
Warning
For external use only
Flammable: Keep away from fire or flame and heated surfaces
Do not use
- if you are allergic to any ingredient
- on open wounds, damaged, or irritated skin
- with a heating pad or TENS device
- after expiration date
When using this product
- do not bandage tightly
- use only as directed
- avoid contact with eyes or mucous
Directions for adults and children 12 years of age and over
- shake well
- apply to affected area not more than 4 times daily
- massage painful area until absorbed into skin
- wash hands after each use with cold water
Children under 12 years of age: consult a doctor
Inactive Ingredients
Aloe barbadensis Leaf (Aloe Vera) Juice, Arnica Montana Flower Extract, Carbomer, Carthamus tinctorious (Safflower) Seed Oil, Cetyl Alcohol, Deionized Water, Dimethyl Sulfone, Ethylhexylglycerin, Glucosamine Sulfate - Glucosamine Sulfate Potassium Chloride, Glycerin, Hemp Extract, Hempseed Oil, Olea europaea (Olive) Oil, Phenoxyethanol, Polysorbate 20, Salix alba, Phenoxyethanol, Polysorbate 20, Salix alba (Willow) Bark Extract, Symphytum Officinale (Comfrey) Leaf Extract
| CONQUER PAIN RELIEF CREAM
menthol, unspecified form lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Steuart Contract Packaging (116952121) |