[de-listing] TIESO FOOT (Foot spray) : 69978-0169-1
TIESO FOOT by
Drug Labeling and Warnings
TIESO FOOT by is a Otc medication manufactured, distributed, or labeled by ANDIVA Inc., ATEC Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TIESO FOOT- benzalkonium chloride, menthol spray
ANDIVA Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
[de-listing] TIESO FOOT (Foot spray) : 69978-0169-1
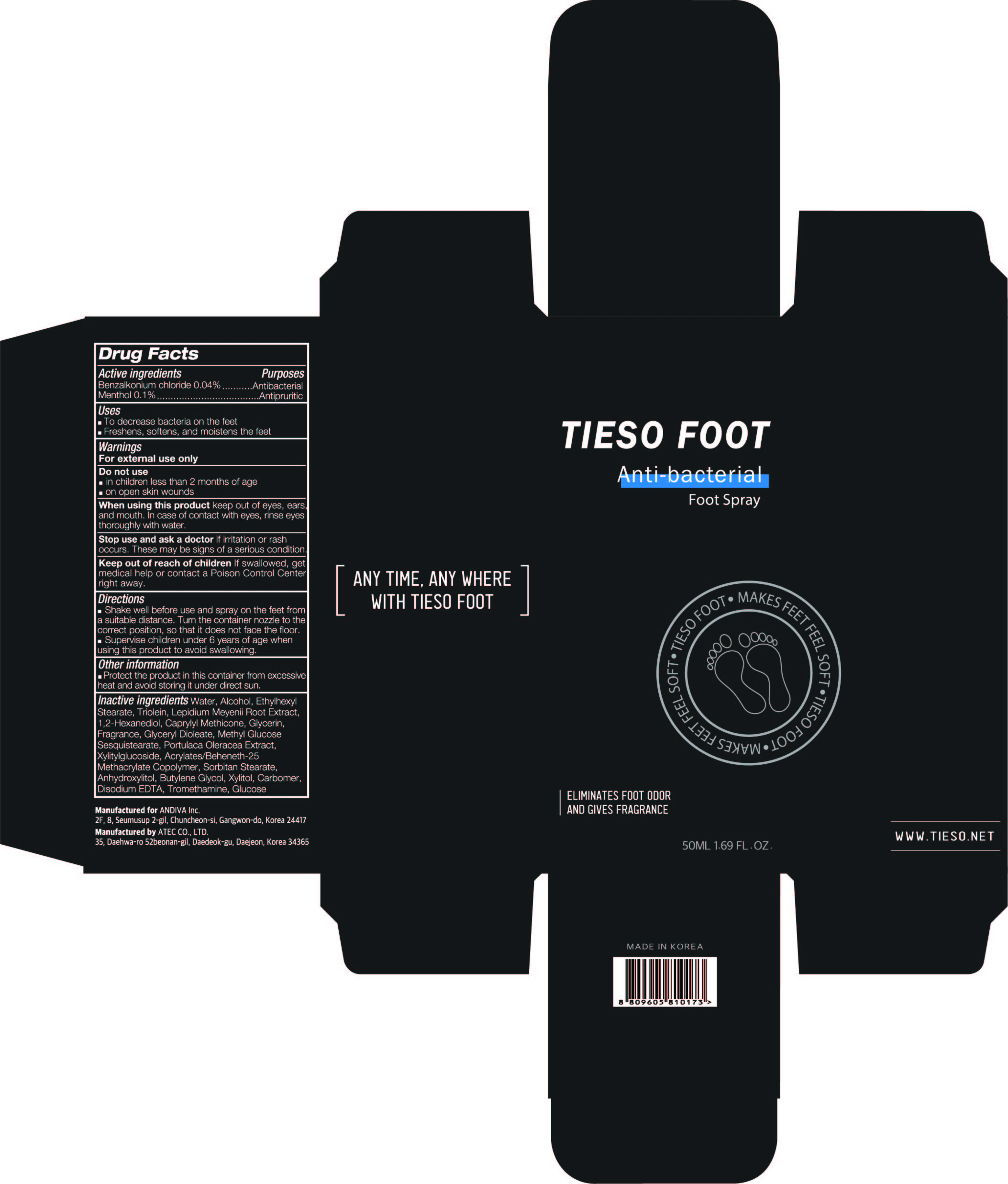
Active ingredients Purposes
Benzalkonium chloride 0.04%..........Antibacterial
Menthol 0.1%.....................................Antipruritic
Inactive ingredients Water, Alcohol, Ethylhexyl
Stearate, Triolein, Lepidium Meyenii Root Extract,
1,2-Hexanediol, Caprylyl Methicone, Glycerin,
Fragrance, Glyceryl Dioleate, Methyl Glucose
Sesquistearate, Portulaca Oleracea Extract,
Xylitylglucoside, Acrylates/Beheneth-25
Methacrylate Copolymer, Sorbitan Stearate,
Anhydroxylitol, Butylene Glycol, Xylitol, Carbomer,
Disodium EDTA, Tromethamine, Glucose
| TIESO FOOT
benzalkonium chloride, menthol spray |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ANDIVA Inc. (695032533) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ATEC Co., Ltd. | 689276681 | manufacture(73184-0169) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.