Lotion 99+ Antiseptic Hand Sanitizer Lotion
Antiseptic Hand Sanitizer by
Drug Labeling and Warnings
Antiseptic Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by OrganiQ Amenities Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC HAND SANITIZER- benzalkonium chloride lotion
OrganiQ Amenities Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lotion 99+ Antiseptic Hand Sanitizer Lotion
Uses
- Hand sanitizer lotion to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Directions
- Adults and children over 2 years:
- For occasional and personal domestic use.
- Supervise children when they use this product.
- Rub thoroughly into hands for at least 30 seconds. Allow to dry.
Inactive ingredients
Aqua/Water/Eau, Cetearyl Alcohol, Butylene Glycol, Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Stearyl Alcohol, Glycerin, Polysorbate 60, Dimethicone, Behentrimonium Methosulfate, Phenoxyethanol, Parfum (Fragrance), Simmondsia Chinensis (Jojoba) Seed Oil, Hydrolyzed Soy Protein, Tocopheryl Acetate, Disodium EDTA, Ethylhexylglycerin, Butylphenyl Methylpropional, Hexyl Cinnamal,
Linalool, Benzyl Salicylate, Alpha-Isomethyl Ionone, Canola Oil, Citronellol, Geraniol, Limonene, Aloe Barbadensis Leaf Juice
| ANTISEPTIC HAND SANITIZER
benzalkonium chloride lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - OrganiQ Amenities Inc. (204248020) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
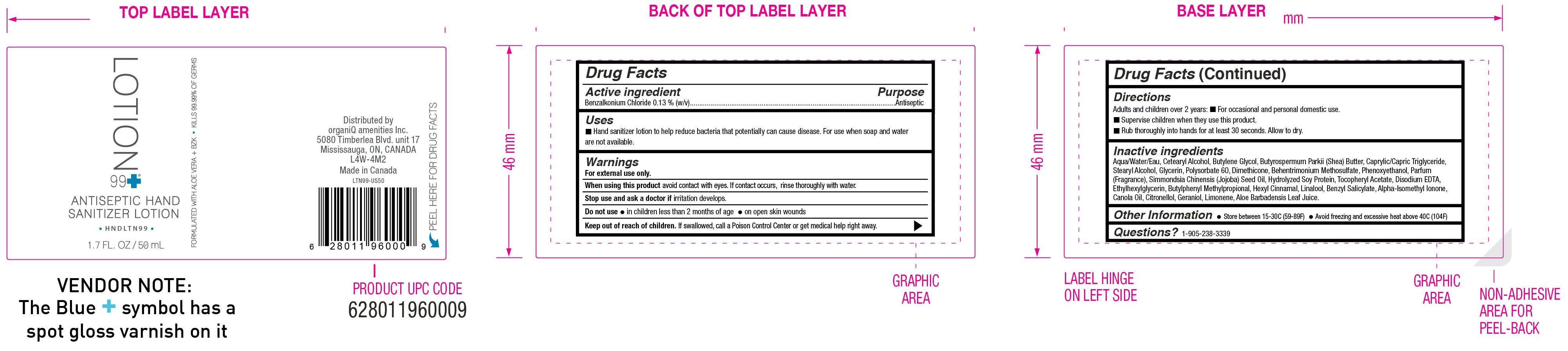 Package Labeling: 50ml
Package Labeling: 50ml
