NALOXONE HYDROCHLORIDE INJECTION, USP, AUTO-INJECTOR- naloxone hydrochloride injection, solution
Naloxone Hydrochloride Injection, USP, Auto-Injector by
Drug Labeling and Warnings
Naloxone Hydrochloride Injection, USP, Auto-Injector by is a Prescription medication manufactured, distributed, or labeled by Kaleo, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NALOXONE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for NALOXONE HYDROCHLORIDE INJECTION.
NALOXONE HYDROCHLORIDE injection, for intramuscular or subcutaneous use
Initial U.S. Approval: 1971INDICATIONS AND USAGE
NALOXONE HYDROCHLORIDE injection is an opioid antagonist indicated for use by military personnel and chemical incident responders for:
- Emergency treatment of patients 12 years of age and older where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected. (1)
- Temporary prophylaxis of respiratory and/or central nervous system depression in military personnel and chemical incident responders entering an area contaminated with high-potency opioids such as fentanyl analogues. (1)
DOSAGE AND ADMINISTRATION
- Administer NALOXONE HYDROCHLORIDE injection according to the INSTRUCTIONS FOR USE. (2.1)
- Administer as soon as possible after known or suspected opioid exposure and seek emergency medical assistance immediately after the first dose. (2.1)
- Administer into the anterolateral aspect of the thigh, through clothing if necessary. (2.2)
- If the patient relapses into respiratory or central nervous system depression after the first dose, administer additional naloxone HCl, as necessary, until emergency medical assistance becomes available. (2.2)
- Keep the patient under continued surveillance until medical care is available. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/0.4 mL solution in a single-dose, pre-filled auto-injector. (3)
CONTRAINDICATIONS
Hypersensitivity to naloxone hydrochloride or to any of the other ingredients in NALOXONE HYDROCHLORIDE injection. (4)
WARNINGS AND PRECAUTIONS
- Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may cause abrupt opioid withdrawal. Monitor for the development of opioid withdrawal. Use of a product that delivers a dose lower than 10 mg of naloxone HCl may be preferable in treatment of a patient with known opioid dependence. (5.1)
- Risk of Recurrent Respiratory and Central Nervous System Depression: Due to the duration of action of naloxone HCl relative to the opioid, keep the patient under continued surveillance and administer additional naloxone HCl, as necessary, while awaiting emergency medical assistance. (5.2)
ADVERSE REACTIONS
The following adverse reactions were observed in more than one subject in clinical studies evaluating NALOXONE HYDROCHLORIDE injection: dizziness, feeling hot, headache, and injection site erythema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact kaleo, Inc. at 1-877-341-5330 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Revised: 2/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing in Adults and Pediatric Patients at Least 12 Years Old
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Precipitation of Severe Opioid Withdrawal
5.2 Risk of Recurrent Respiratory and Central Nervous System Depression
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
NALOXONE HYDROCHLORIDE injection is indicated for use by military personnel and chemical incident responders for:
- Emergency treatment of patients 12 years of age and older where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected.
- Temporary prophylaxis of respiratory and/or central nervous system depression in military personnel and chemical incident responders entering an area contaminated with high-potency opioids such as fentanyl analogues.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Read the Instructions for Use at the time of receiving a single-dose, pre-filled NALOXONE HYDROCHLORIDE injection auto-injector.
- Administer NALOXONE HYDROCHLORIDE injection as soon as possible after known or suspected opioid exposure because prolonged respiratory depression may result in damage to the central nervous system or death.
- NALOXONE HYDROCHLORIDE injection contains 10 mg/0.4 mL naloxone HCl in a single-dose, pre-filled auto-injector for intramuscular or subcutaneous use only.
- Do not attempt to reuse the NALOXONE HYDROCHLORIDE injection auto-injector.
- Periodically visually inspect NALOXONE HYDROCHLORIDE injection through the viewing window for particulate matter. Request a replacement if the solution is cloudy or contains particles, or if the glass container is damaged.
- Once the red safety guard is removed, NALOXONE HYDROCHLORIDE injection auto-injector must be used immediately or disposed of properly. Do not attempt to replace the red safety guard once it is removed.
2.2 Dosing in Adults and Pediatric Patients at Least 12 Years Old
- Inject NALOXONE HYDROCHLORIDE injection into the anterolateral aspect of the thigh according to the Instructions for Use and the printed instructions on the device label.
- In summary,
- Ensure that the injection site is free of other materials (e.g., equipment or other obstructions), except for clothing.
- Pull the auto-injector from the outer case.
- When ready to use, firmly pull off the red safety guard. Do not touch the black base of the auto-injector, which is where the needle comes out.
- Place the black end of the auto-injector against the anterolateral aspect of the thigh, through clothing, if needed.
- Press firmly until you hear a click and hiss sound and then hold in place for 5 seconds.
- Upon actuation, the auto-injector automatically inserts the needle intramuscularly or subcutaneously, delivers the naloxone HCl injection, and retracts the needle into the device. The needle will not be visible before, during, or after the injection.
- Post-injection, the black base locks in place and a red indicator appears in the drug viewing window.
- NALOXONE HYDROCHLORIDE injection can be injected through clothing or MOPP4 PPE.
- NALOXONE HYDROCHLORIDE injection can be self- or buddy-administered.
Emergency treatment of patients 12 years of age and older where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected.
- Seek immediate emergency medical assistance immediately after administration of the first dose of NALOXONE HYDROCHLORIDE injection.
- Keep the patient under continued surveillance until medical care is available.
- If the patient relapses into respiratory and/or central nervous system depression after the first dose of NALOXONE HYDROCHLORIDE injection, administer additional naloxone HCl, as necessary, until emergency medical assistance becomes available.
- If the patient does not show some improvement after administering the dose of NALOXONE HYDROCHLORIDE injection, consider if the respiratory depression is due to a non-opioid etiology.
Temporary prophylaxis of respiratory and/or central nervous system depression in military personnel and chemical incident responders entering an area contaminated with high-potency opioids such as fentanyl analogues.
- Administer immediately prior to entering an area believed to be contaminated with high-potency opioids.
- Each pre-filled NALOXONE HYDROCHLORIDE injection can only be used one time.
- NALOXONE HYDROCHLORIDE injection provides temporary protection. If exposure to high potency opioids is prolonged, additional doses may be necessary.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Precipitation of Severe Opioid Withdrawal
The safety of NALOXONE HYDROCHLORIDE injection, 10 mg, in an auto-injector has not been established in opioid-dependent adults. Because NALOXONE HYDROCHLORIDE injection is expected to cause abrupt opioid withdrawal in opioid-dependent individuals, use NALOXONE HYDROCHLORIDE injection when the potential benefits are expected to outweigh the risks. Use of a product that delivers a dose lower than 10 mg naloxone HCl may be preferable in treatment of a patient with known opioid dependence.
Abrupt reversal of opioid depression after using naloxone HCl may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Although a direct cause and effect relationship has not been established, after use of naloxone HCl, monitor individuals with opioid-dependence for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone HCl is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
5.2 Risk of Recurrent Respiratory and Central Nervous System Depression
The duration of action of most opioids may exceed that of naloxone HCl resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek emergency medical assistance immediately after delivering the first dose of NALOXONE HYDROCHLORIDE injection. Keep the patient under continued surveillance and administer additional naloxone HCl as necessary [see Dosage and Administration (2.2)]. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.1)]
- Recurrent Respiratory and Central Nervous System Depression [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In three pharmacokinetic studies with a total of 78 healthy adult subjects exposed to NALOXONE HYDROCHLORIDE injection 0.4 mg auto-injector, two NALOXONE HYDROCHLORIDE injection 0.4 mg (0.8 mg naloxone HCl total) auto-injectors, NALOXONE HYDROCHLORIDE injection 2 mg auto-injector, or NALOXONE HYDROCHLORIDE injection 10 mg auto-injector, adverse reactions occurring in more than one subject were dizziness, feeling hot, headache, and injection site erythema.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the use of naloxone HCl to reverse the effects of opioids. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: aggression, anger, anxiety, back pain, bradycardia, cardiac arrest, confusion, depression, disorientation, dyspnea, hypertension, hypotension, loss of consciousness, malaise, miosis, pain, pulmonary edema, somnolence, unresponsiveness to stimuli, and ventricular tachycardia and fibrillation. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone HCl have resulted in significant reversal of analgesia and have caused agitation [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Life-sustaining therapy for opioid overdose should not be withheld (see Clinical Considerations). There is an absence of data on naloxone HCl administered for known or suspected opioid overdose in pregnant patients. Available data from retrospective cohort studies on oral naloxone use in pregnant women for opioid use disorder have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, no embryotoxic or teratogenic effects were observed in mice and rats treated with naloxone HCl during the period of organogenesis at doses equivalent to 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg naloxone HCl [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Disease-associated maternal and/or embryo/fetal risk
An opioid overdose is a medical emergency and can be fatal for the pregnant woman and fetus if left untreated. Treatment with NALOXONE HYDROCHLORIDE injection for opioid overdose should not be withheld because of potential concerns regarding the effects of NALOXONE HYDROCHLORIDE injection on the fetus.
Animal Data
Naloxone HCl was administered during organogenesis to mice and rats at doses 4-times and 8-times, respectively, the dose of 10 mg/day given to a 50 kg human (when based on body surface area or mg/m2). These studies demonstrated no embryotoxic or teratogenic effects due to naloxone HCl.
8.2 Lactation
Risk Summary
Naloxone is minimally orally available and is unlikely to affect the breastfed infant. There is no information regarding the presence of naloxone in human milk, or the effects of naloxone on the breastfed infant or on milk production. Published studies in lactating women have shown that naloxone does not affect prolactin or oxytocin hormone levels.
8.4 Pediatric Use
The safety and effectiveness of NALOXONE HYDROCHLORIDE injection, 10 mg, in an auto-injector have been established in pediatric patients 12 years of age and older for emergency treatment where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected.
Use of NALOXONE HYDROCHLORIDE injection in this age group is supported by evidence from adult bioavailability studies, adult PK/PD modeling and simulation analysis supporting an effective dose, along with additional evidence from the reported safe and effective use of other naloxone HCl products [see Clinical Pharmacology (12.2)]. No pediatric studies were conducted for NALOXONE HYDROCHLORIDE injection.
The safety and effectiveness of NALOXONE HYDROCHLORIDE injection for intramuscular and subcutaneous use have not been established for pediatric patients less than 12 years old. Absorption of naloxone HCl following subcutaneous or intramuscular administration in pediatric patients may be erratic or delayed. Even when the opiate-intoxicated pediatric patient responds appropriately to naloxone HCl injection, the patient must be carefully monitored for at least 24 hours as a relapse may occur as naloxone is metabolized.
In opioid-dependent pediatric patients, administration of naloxone HCl may result in an abrupt and complete reversal of opioid effects, precipitating an acute opioid withdrawal syndrome. In these settings where it may be preferable to avoid the abrupt precipitation of acute opioid withdrawal symptoms, consider use of an alternative naloxone HCl product that can be dosed according to weight and titrated to effect [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of naloxone HCl did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Geriatric patients have a greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Therefore, the systemic exposure of naloxone can be higher in these patients.
-
11 DESCRIPTION
NALOXONE HYDROCHLORIDE injection, USP, for intramuscular or subcutaneous use, is an opioid antagonist in a pre-filled, single-dose auto-injector containing 10 mg of naloxone HCl in 0.4 mL solution. NALOXONE HYDROCHLORIDE injection is not made with natural rubber latex. Chemically, naloxone HCl is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride with the following structure:
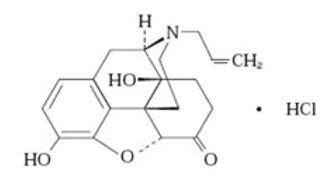
C19H21NO4HC1
M.W.363.84
Naloxone HCl occurs as a white to slightly off-white powder, and is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
Each 0.4 mL of NALOXONE HYDROCHLORIDE injection contains 10 mg of naloxone HCl (equivalent to 9.0 mg naloxone base), 3.34 mg of sodium chloride, hydrochloric acid to adjust pH, and water for injection. The pH range is 3.0 to 4.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Naloxone HCl is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone HCl reverses the effects of opioids, including respiratory depression, sedation, and hypotension. Also, it can reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2 Pharmacodynamics
When naloxone HCl is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration.
The duration of action is dependent upon the dose and route of administration of naloxone HCl.
Simulations based on data from healthy volunteer studies demonstrated that a single 10 mg naloxone HCl dose could improve depressed ventilation induced by morphine, buprenorphine, fentanyl, and carfentanil. The ability to reverse decreased ventilation rate after naloxone HCl treatment was dependent on the administered opioid, amount of opioid, amount of naloxone, and timing of naloxone HCl administration relative to opioid exposure.
12.3 Pharmacokinetics
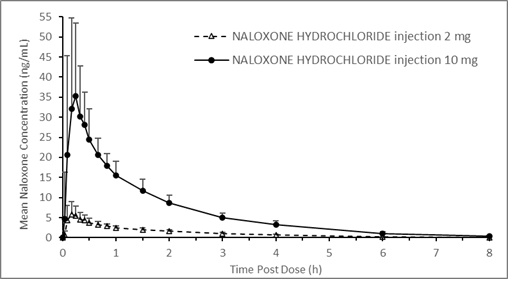
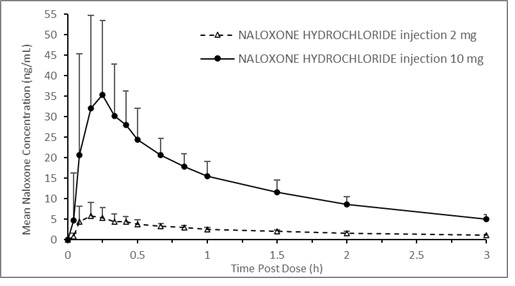
A pharmacokinetic study using a crossover design was conducted in 24 fasted healthy subjects to evaluate a single 2 mg NALOXONE HYDROCHLORIDE injection (0.4 mL of 5 mg/mL naloxone HCl solution) and a single 10 mg NALOXONE HYDROCHLORIDE injection (0.4 mL of 25 mg/mL naloxone HCl solution) administered to the anterolateral aspect of the thigh. Plasma samples were analyzed for naloxone 12-hours post dose. The pharmacokinetic parameters obtained in this study are shown in Table 1 and the plasma concentration time profiles of naloxone are presented in Figure 1. A slightly greater than 5-fold increase was observed for 10 mg NALOXONE HYDROCHLORIDE injection compared to 2 mg NALOXONE HYDROCHLORIDE injection for the mean pharmacokinetic parameters of Cmax, AUC0-t, and AUC0-inf.
Table 1 Mean Pharmacokinetic Parameters (%CV) for Naloxone Following NALOXONE HYDROCHLORIDE injection Intramuscular/Subcutaneous Administration to Healthy Subjects
† Tmax reported as median (minimum, maximum) Parameter 2 mg NALOXONE
HYDROCHLORIDE
injection (N=24)10 mg NALOXONE
HYDROCHLORIDE
injection (N=24)Tmax (h)† 0.17 (0.08, 0.67) 0.26 (0.09, 0.67) Cmax (ng/mL) 6.95 (44.8) 42.0 (49.9) AUC0-t (ng.h/mL) 9.22 (14.6) 51.1 (17.9) AUC0-inf (ng.h/mL) 9.25 (14.6) 51.3 (17.8) T1/2(h) 1.46 (11.1) 1.46 (14.1) Figure 1 Mean ± SD Plasma Concentration of Naloxone, (a) 0-8 h and (b) 0-3 h Following Intramuscular/Subcutaneous Administration using NALOXONE HYDROCHLORIDE injection
(a)
(b)
Absorption
The median Tmax for NALOXONE HYDROCHLORIDE injection is 15.6 minutes (range 5.4 to 40.2 minutes).
Distribution
Following parenteral administration, naloxone is distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent, but significant binding of naloxone also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.
Elimination
The mean plasma half-life of naloxone in healthy adults was 1.46 hours (14.1%CV) following a single administration of NALOXONE HYDROCHLORIDE injection.
Metabolism
Naloxone HCl is metabolized in the liver, primarily by glucuronide conjugation with naloxone-3-glucoronide as the major metabolite.
Excretion
After an oral or intravenous dose, about 25-40% of naloxone is excreted as metabolites in urine within 6 hours, about 50% in 24 hours, and 60-70% in 72 hours.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been completed.
Mutagenesis
Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study.
Impairment of Fertility
Reproduction studies conducted in mice and rats at doses 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg/day (when based on surface area or mg/m2), demonstrated no adverse effect of naloxone HCl on fertility.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
NALOXONE HYDROCHLORIDE injection, a colorless to yellow solution of 10 mg/0.4 mL naloxone HCl, provided as follows:
NDC: 60842-002-02: Package containing ten single-dose 10 mg/0.4 mL auto-injectors
16.2 Storage and Handling
Storage and Shipping
Store at controlled room temperature 15°C - 25°C (59°F - 77°F); excursions permitted between 4°C and 40°C (39°F and 104°F). Do not freeze. Protect from heat.
Use and Handling
Store the NALOXONE HYDROCHLORIDE injection auto-injector in the outer case provided.
If NALOXONE HYDROCHLORIDE injection is frozen and is needed in an emergency, do NOT wait for NALOXONE HYDROCHLORIDE injection to thaw; get emergency medical help right away. Once thawed, NALOXONE HYDROCHLORIDE injection may be used.
Manufactured for:
Kaleo, Inc.
Richmond, VA 23219This product may be covered by one or more U.S. patents or pending patent applications. See www.kaleopharma.com/pat for details.
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
NALOXONE HYDROCHLORIDE injection (Nuh-laak-sown hai-drow-klaw-ride)
for intramuscular or subcutaneous useRead the Instructions for Use that comes with NALOXONE HYDROCHLORIDE injection before using it.
NALOXONE HYDROCHLORIDE injection comes in a single-dose, pre-filled auto-injector.
NALOXONE HYDROCHLORIDE injection is indicated for use by military personnel and chemical incident responders for:
- Emergency treatment of people 12 years of age and older where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected.
- Temporary prevention (prophylaxis) of either breathing problems (respiratory depression), decreased brain and nerve function (central nervous system depression), or both in military personnel and chemical incident responders entering an area contaminated with high-potency opioids such as fentanyl analogues.
Important:
- Administer NALOXONE HYDROCHLORIDE injection as soon as possible after known or suspected opioid exposure.
- If an individual is unresponsive and opioid exposure is suspected, administer NALOXONE HYDROCHLORIDE injection as quickly as possible because an opioid emergency can cause severe injury or death.
- Each NALOXONE HYDROCHLORIDE injection auto-injector can only be used 1 time.
You do not need to assemble your auto-injector. It comes already assembled for use.
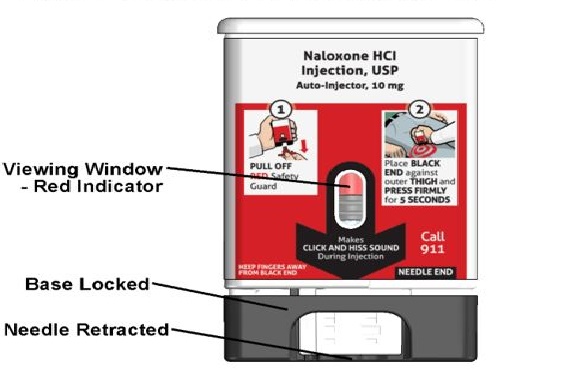
Figure A – NALOXONE HYDROCHLORIDE injection auto-injector Parts

Important note: Before you use the NALOXONE HYDROCHLORIDE injection auto-injector, look at the viewing window. Do not use the auto-injector if the medicine is cloudy or contains particles or if there is a red indicator in the viewing window. Use a new NALOXONE HYDROCHLORIDE injection auto-injector.
How to use the auto-injector:
Step 1.
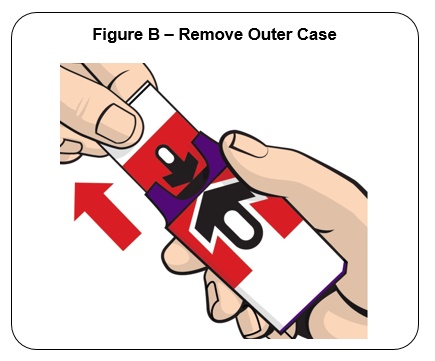
Firmly pull the auto-injector from the outer case. See Figure B .
Do not go to Step 2 (Do not remove the Red safety guard) until you are ready to use the auto-injector. If you are not ready to use the auto-injector, put it back in the outer case for later use.
If NALOXONE HYDROCHLORIDE injection auto-injector is frozen, do not wait for it to thaw. Use another auto-injector if available. If another auto-injector is not available, get emergency medical help right away.
NALOXONE HYDROCHLORIDE injection auto-injector may still be used if it has been thawed after being previously frozen.
Step 2.
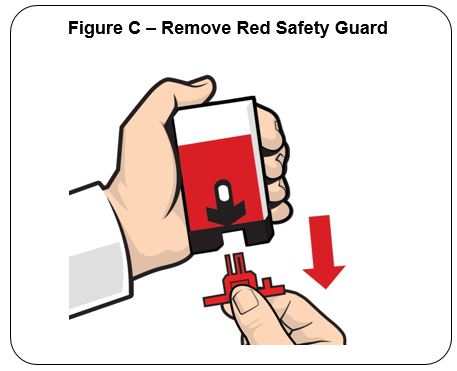
Pull off the Red safety guard. See Figure C .
To reduce the chance of an accidental injection, do not touch the Black base of the auto-injector, which is where the needle comes out
Note: The Red safety guard is made to fit tightly. Pull firmly to remove.
Do not replace the Red safety guard after it is removed.
Step 3.
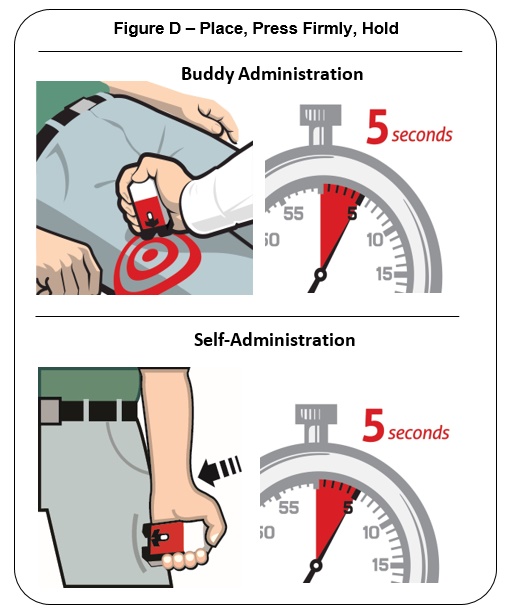
Place the Black end of the auto-injector against the outer thigh, through clothing or personal protective equipment (including MOPP4 PPE), if needed. Make sure that the injection site is free of other materials such as equipment or other obstructions, except for clothing. NALOXONE HYDROCHLORIDE injection auto-injector may be self-administered or administered by a buddy.
Press firmly until you hear a click and hiss sound and then hold in place for 5 seconds. See Figure D .
Note: The auto-injector makes a distinct sound (click and hiss) when it is pressed against the thigh. The click and hiss indicates the start of the injection. This is normal and means that the auto-injector is working correctly. Keep the auto-injector firmly pressed against the thigh for 5 seconds after you hear the click and hiss sound. After the medicine is injected, the needle will retract back up into the auto-injector and is not visible after use.
Step 4.
After administering the first dose of NALOXONE HYDROCHLORIDE injection, get emergency medical help right away.
Rescue breathing or CPR (cardiopulmonary resuscitation) may be given while waiting for emergency medical help. If there is no response to NALOXONE HYDROCHLORIDE injection consider if respiratory depression is caused by something other than opioid exposure. Watch the person closely. If symptoms return, additional naloxone may be administered.
You may need additional doses of NALOXONE HYDROCHLORIDE injection if you are being exposed to high-potency opioids for an extended period of time. NALOXONE HYDROCHLORIDE injection auto-injector provides temporary protection against opioid exposure.
How to know that the auto-injector has been used.. See Figure E .
- The viewing window will no longer be clear. You will see a Red indicator.
- The Black base will lock into place.
Figure E - NALOXONE HYDROCHLORIDE injection auto-injector – After Use
What to do after the auto-injector has been used:
- Get emergency medical help right away.
- The auto-injector cannot be reused. Put the auto-injector back in the outer case after it has been used.
- Do not throw away the auto-injector in the trash. Do not recycle the auto-injector.
- The auto-injector must be properly disposed of in a sharps container.
There may be local or state laws about how to throw away used auto-injectors.
How should I store NALOXONE HYDROCHLORIDE injection?
- Store NALOXONE HYDROCHLORIDE injection auto-injector at room temperature between 59°F to 77°F (15°C to 25°C). Storage temperature excursions permitted between 39°F to 104°F (4°C to 40°C).
- Protect from heat and do not freeze.
- Keep the NALOXONE HYDROCHLORIDE injection auto-injector in its outer case until ready to use.
- Occasionally check the viewing window of the NALOXONE HYDROCHLORIDE injection auto-injector. If the medicine is cloudy, contains particles, or if the glass container is damaged, replace the auto-injector with a new one.
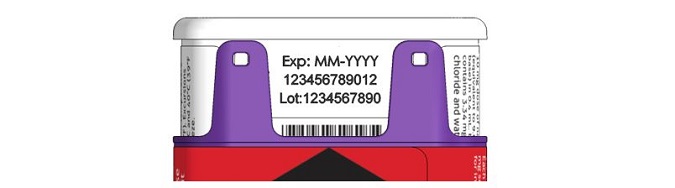
- NALOXONE HYDROCHLORIDE injection auto-injector has an expiration date. See Figure F . Replace it before the expiration date.
Figure F – Expiration Date Located on Back of the NALOXONE HYDROCHLORIDE injection auto-injector
Keep NALOXONE HYDROCHLORIDE injection and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Kaleo, Inc., Richmond, VA 23219
Issued: 02/2022 - PRINCIPAL DISPLAY PANEL - NDC: 60842-002-02 - 10 mg Auto-injector Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 60842-002-02 - Device Label
- PRINCIPAL DISPLAY PANEL - NDC: 60842-002-02 - Outer Case Label
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE INJECTION, USP, AUTO-INJECTOR
naloxone hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60842-002 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 10 mg in 0.4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60842-002-02 10 in 1 CARTON 02/28/2022 1 0.4 mL in 1 DOSE PACK; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215457 02/28/2022 Labeler - Kaleo, Inc. (182938485)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.