STAMARIL- yellow fever virus strain 17d-204 live antigen kit
STAMARIL by
Drug Labeling and Warnings
STAMARIL by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc., Sanofi Pasteur SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PATIENT PACKAGE INSERT
Yellow fever vaccine (Live)
Read all of this leaflet carefully before you or your child are vaccinated because it contains important information for you. - Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This vaccine has been prescribed for you or your child only. Do not pass it on to others.
- If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
- What STAMARIL is and what it is used for
- What you need to know before you use STAMARIL
- How to use STAMARIL
- Possible side effects
- How to store STAMARIL
- Contents of the pack and other information.
1. WHAT STAMARIL IS AND WHAT IT IS USED FOR
Pharmacotherapeutic group: Yellow Fever Vaccine (Live), ATC code: J07B-L01.
STAMARIL is a vaccine that provides protection against a serious infectious disease called yellow fever.
Yellow fever occurs in certain areas of the world and is spread to man through the bites of infected mosquitoes.
STAMARIL is given to people who:
- are travelling to, passing through or living in an area where yellow fever occurs,
- are travelling to any country that requires an International Certificate of Vaccination for entry (this may depend on the countries previously visited during the same trip),
- may handle infectious materials such as laboratory workers.
To obtain a valid vaccination certificate against yellow fever, it is necessary to be vaccinated in an approved vaccination centre so that an International Certificate of Vaccination can be issued. This certificate is valid from 10 days after the first dose of vaccine. When a booster is needed, the certificate (see section 3) is valid immediately after the injection.
2. WHAT YOU NEED TO KNOW BEFORE YOU OR YOUR CHILD USE STAMARIL
It is important to tell your doctor or nurse if any of the points below apply to you or your child. If there is anything you do not understand, ask your doctor or nurse to explain.
Do not use STAMARIL if you or your child:
- are allergic to:
- the active substance, or
- any of the other ingredients of this vaccine listed in section 6, or
- eggs or chicken proteins,
- have experienced a severe allergic reaction after a previous dose of any yellow fever vaccine,
- is less than 6 months old,
- have a poor or weakened immune system for any reason, such as illness or medical treatments (for example corticoids or chemotherapy),
- have a weakened immune system due to HIV infection. Your doctor will tell you if you can still receive STAMARIL based on the results of your blood tests,
- are infected with HIV and have active symptoms due to the infection,
- have a history of problems with your thymus gland or have had your thymus gland removed for any reason,
- have an illness with a high or moderate temperature or an acute illness. The vaccination will be postponed until you have recovered.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using STAMARIL.
- If you are over 60 years old or if your child is less than 9 months as you have an increased risk of certain types of severe but rare reactions to vaccines (including serious reactions that affect the brain and nerves, as well as vital organs, see section 4). You will only be given the vaccine if the risk of infection with the virus is well established in countries where you are going to stay,
- If your child is aged 6 to 9 months. STAMARIL may be given to children aged between 6 and 9 months only in special situations and on the basis of current official advice,
- If you or your child are infected by the HIV virus but do not have active symptoms due to the infection. Your doctor will advise if STAMARIL can be given based on the results of laboratory tests and specialist advice,
- If you or your child have any bleeding disorders (such as haemophilia or a low level of platelets) or are taking any medicines that stop the blood clotting normally. You can still be given STAMARIL provided that it is injected under the skin and not into muscle (see section 3).
As with all vaccines, STAMARIL may not fully protect all persons who are vaccinated.
Fainting can occur following, or even before, any needle injection. Therefore tell your doctor or nurse if you or your child fainted with a previous injection.
Other medicines and STAMARIL
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you have recently had any treatment or medicine which may have weakened your immune system, the vaccination must be delayed until your laboratory results show that your immune system has recovered. Your doctor will advise you when it is safe for you to be vaccinated.
STAMARIL can be given at the same time as measles vaccine or vaccines against typhoid fever (those containing the Vi capsular polysaccharide) and/or hepatitis A.
Vaccination with STAMARIL may lead to false positive results of blood tests for dengue or Japanese encephalitis. If you or your child have in the future such tests prescribed, please inform your doctor about this vaccination.
Pregnancy and breast-feeding
If you are pregnant, or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before being vaccinated.
You should not receive STAMARIL unless this cannot be avoided. Your doctor or pharmacist can advise you on whether it is essential that you are vaccinated while pregnant or breast-feeding.
Posology
STAMARIL is given as a single, 0.5 millilitre dose to adults and children from 6 months of age.
The first dose should be given at least 10 days before protection from yellow fever is needed. This is because it takes 10 days for the first dose of vaccine to work and provide good protection against the yellow fever virus. The protection provided by this dose is expected to last at least 10 years and may be life-long.
A booster with one dose (0.5 millilitre) may be needed:
- if you or your child had an insufficient response to the first dose,
- or after at least 10 years if it is required as a condition of entry in some countries.
How STAMARIL is given
STAMARIL is given as an injection by a doctor or nurse. It is usually injected just underneath the skin but it can be given into a muscle.
It must not be injected into a blood vessel.
If you or your child use more STAMARIL than you should
In some cases, more than the recommended dose was used.
In these cases, when side effects were reported, the information was in line with what is described in section 4.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist or nurse.
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Serious side effects
The following serious side effects have sometimes been reported:
Allergic reactions:
- Rash, itching or hives on the skin.
- Swelling of the face, lips, tongue or other parts of the body.
- Difficulty swallowing or breathing.
- Loss of consciousness.
Reactions affecting the brain and nerves:
These may occur within one month of the vaccination and have sometimes been fatal.
Symptoms include:
- High fever with headache and confusion.
- Extreme tiredness.
- Stiff neck.
- Inflammation of brain and nerve tissues.
- Fits.
- Loss of movement or feeling in part or all of the body (Guillain-Barré Syndrome or focal neurological deficit).
Serious reaction affecting vital organs:
This may occur within 10 days of the vaccination and may have a fatal outcome. The reaction can resemble an infection with the yellow fever virus. It generally begins with feeling tired, fever, headache, muscle pain and sometimes low blood pressure. It may then go on to severe muscle and liver disorders, drops in number of some types of blood cells resulting in unusual bruising or bleeding and increased risk of infections, and loss of normal functioning of the kidneys and lungs.
If you experience ANY of the above symptoms contact your doctor IMMEDIATELY.
Other side effects
Very common (may affect more than 1 in 10 people):
- Headache
- Mild or moderate tiredness or weakness (asthenia).
- Pain or discomfort at the injection site.
- Muscle pains.
- Fever (in children).
- Vomiting (in children).
Common (may affect up to 1 in 10 people):
- Fever (in adults).
- Vomiting (in adults).
- Painful joints.
- Feeling sick (nausea).
- Reactions at the injection site: redness, bruising, swelling or appearance of a hard lump.
Uncommon (may affect up to 1 in 100 people):
- Dizziness.
- Stomach pains.
- A pimple (papule) at the injection site.
Rare (may affect up to 1 in 1,000 people):
- Diarrhoea.
- Runny, blocked or itchy nose (rhinitis).
Not known (frequency cannot be estimated from the available data):
- Swollen glands (lymphadenopathy).
- Numbness or pins and needles sensation (paraesthesia).
- Flu-like illness.
Additional side effects in children
Very common (may affect more than 1 in 10 people):
- Irritability, crying.
- Appetite loss.
- Drowsiness.
- These side effects usually occurred within the 3 days following vaccination and lasted usually not more than 3 days. Most of these side effects were of mild intensity.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE STAMARIL
Keep out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C – 8°C). Do not freeze.
Keep the vial of powder and the syringe of solvent in the outer carton in order to protect from light.
Use immediately after reconstitution.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What STAMARIL contains
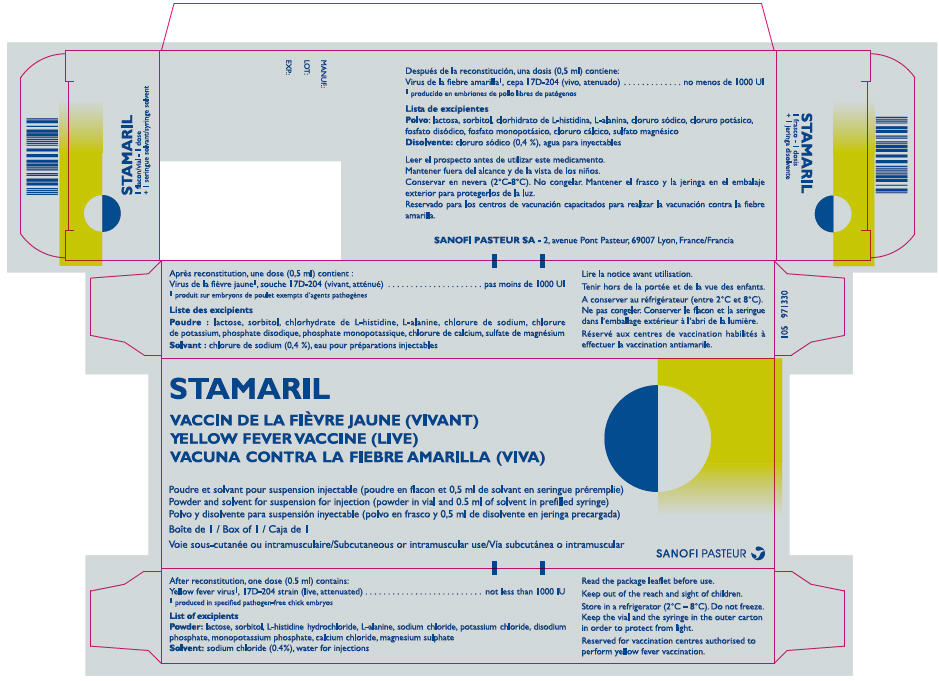
After reconstitution, for one dose (0.5 ml):
- * produced in specified pathogen-free chick embryos.
- The active substance is:
Yellow fever virus*, 17D-204 strain (live, attenuated) not less than 1000 IU - The other ingredients are:
Lactose, sorbitol, L-Histidine hydrochloride, L-Alanine, sodium chloride, potassium chloride, disodium phosphate dihydrate, potassium dihydrogen phosphate, calcium chloride, magnesium sulphate and water for injections.
What STAMARIL is and contents of the pack
STAMARIL is presented as a powder and solvent for suspension for injection (powder in vial (0.5 ml dose) + solvent in pre-filled syringe (0.5 ml dose) with or without needle(s)). Box of 1, 10 or 20.
After reconstitution the suspension is beige to pink beige, more or less opalescent.
Not all pack sizes may be marketed.
Marketing authorisation holder
SANOFI PASTEUR - 14 Espace Henry Vallée - 69007 LYON - FRANCE
This leaflet was last revised in:
02/2018.Other
The following information is intended for healthcare professionals only:
Instructions for reconstitution:
Before use, the beige to orange beige powder is mixed with the clear colourless sodium chloride solvent provided in a syringe to make a beige to pink beige suspension, which is more or less opalescent.
For syringes without attached needle only: after removing the syringe tip cap, a needle should be firmly placed on the tip of the syringe and secured by rotating a quarter of a turn (90°).
The vaccine is reconstituted by adding the solvent provided in the pre-filled syringe to the vial. The vial is shaken and, after complete dissolution, the suspension obtained is withdrawn into the same syringe for injection.
Contact with disinfectants is to be avoided since they may inactivate the virus.
Use immediately after reconstitution.
Before administration, the reconstituted vaccine should be vigorously shaken.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
See also section 3. HOW TO USE STAMARIL.
-
HEALTH CARE PROVIDER LETTER
April 2017
Dear Health Care Professional:
This letter is to provide an update on the status of YF-VAX® (Yellow Fever Vaccine) supply. Sanofi Pasteur is experiencing delays in the production process of YF-VAX vaccine and it is anticipated that the product will be unavailable from mid-2017 to mid-2018 as we transition production to a new state-of-the-art facility. Ordering restrictions have been implemented to responsibly manage the limited remaining supply of YF-VAX vaccine. YF-VAX vaccine will continue to be available while current supplies last.
As the sole manufacturer of yellow fever vaccine in the United States, we take our responsibility seriously and are making every effort to maintain access to yellow fever vaccine. We have worked with the Food and Drug Administration (FDA) to make another yellow fever vaccine available in the US. This vaccine, STAMARIL® (Yellow Fever Vaccine [Live]), manufactured by Sanofi Pasteur in France, is a live, attenuated yellow fever vaccine that is investigational/unlicensed in the US, but it is registered and currently distributed in over 70 countries. The mechanism by which we can make this vaccine available is called an Expanded Access Investigational New Drug Application (IND), made possible under US regulations.
An Expanded Access IND program is similar to a clinical trial and requires training of clinical site staff and monitoring of vaccine recipients. There are additional rigorous documentation and informed consent procedures. Further, only health care providers who are certified by state health departments to administer yellow fever vaccine will be eligible. Given all of these restrictions, a limited number of clinical sites can participate in this program. These locations have been selected to include sites that immunize the most patients with YF-VAX vaccine, and geographic location. The objective is to provide the broadest access possible to yellow fever vaccine, given the regulatory requirements of the Expanded Access IND program.
Once YF-VAX vaccine is no longer available, health care providers and patients will be able to find locations that will administer STAMARIL vaccine by visiting the CDC web page at http://wwwnc.cdc.gov/travel/yellow-fever-vaccination-clinics/search. They may also visit http://wwwnc.cdc.gov/travel/ for information about which countries require yellow fever vaccination for entry and for which countries the CDC recommends yellow fever vaccination.
Supply of YF-VAX vaccine from our new US facility is planned for mid-2018. A significant number of doses had been planned to bridge the gap between the former manufacturing facility and the new facility. However, there was an unavoidable equipment issue that resulted in halting production. This did not affect any doses of YF-VAX vaccine that are being shipped to customers. All doses shipped to customers received release clearance for safety and potency from the FDA Center for Biologics Evaluation and Research.
Sanofi Pasteur recognizes the challenge this supply disruption will cause for your practice and for patients in need of yellow fever vaccine. We are making every effort to see that yellow fever vaccination continues in the US during this YF-VAX vaccine supply disruption and we appreciate your understanding.
For more information on YF-VAX vaccine availability and our vaccine supply situation, you can contact Sanofi Pasteur by calling 1-800-VACCINE (1-800-822-2463).
IMPORTANT SAFETY INFORMATION FOR YF-VAX VACCINE
Indication
YF-VAX vaccine is indicated for active immunization for the prevention of yellow fever in persons 9 months of age and older in the following categories: persons living in or traveling to yellow fever endemic areas, persons traveling internationally through countries with yellow fever, and laboratory personnel who handle virulent yellow fever virus or concentrated preparations of the yellow fever vaccine virus strains.
Safety Information
The most common local and systemic adverse reactions to YF-VAX vaccine include edema and pain at the injection site; mild headache, myalgia, and fever. Other adverse reactions may occur. YF-VAX vaccine should not be administered to an individual with a history of acute hypersensitivity to eggs, egg products, or to any component of the vaccine. Anaphylaxis may occur following the use of YF-VAX vaccine, even in individuals with no prior history of hypersensitivity to the vaccine components. Infants younger than 9 months of age should not be vaccinated with YF-VAX vaccine. Vaccination with YF-VAX vaccine is also contraindicated in lactating women who are providing breast milk to infants younger than 9 months of age due to the potential for transmission of vaccine virus in breast milk. In addition, YF-VAX vaccine is contraindicated in immunosuppressed individuals. Yellow fever vaccine-associated viscerotropic and neurotropic diseases are known rare serious adverse events. Vaccination with YF-VAX vaccine may not protect all individuals.
Before administering YF-VAX vaccine, please click here accompanying full Prescribing Information.
Sincerely,
Matthew Wilcox
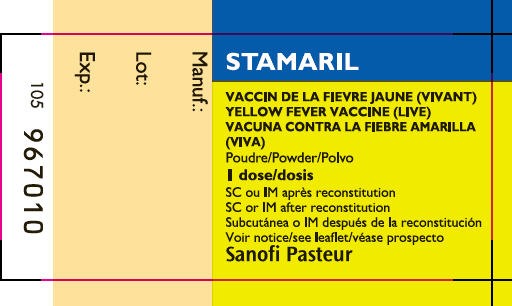
Vice President Marketing, US - PRINCIPAL DISPLAY PANEL - 0.5 ml Vial Label
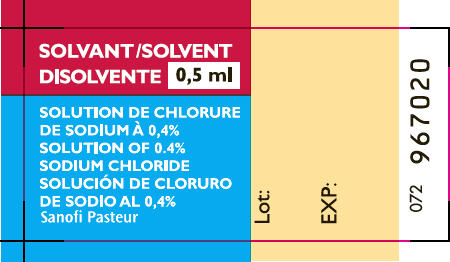
- PRINCIPAL DISPLAY PANEL - 0.5 ml Syringe Label
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
STAMARIL
yellow fever virus strain 17d-204 live antigen kitProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-913 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-913-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 0.5 mL Part 2 1 SYRINGE 0.5 mL Part 1 of 2 STAMARIL
yellow fever virus strain 17d-204 live antigen injection, powder, lyophilized, for suspensionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN (UNII: PY4EET359T) (YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN - UNII:PY4EET359T) YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN 1000 [iU] in 0.5 mL Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SORBITOL (UNII: 506T60A25R) HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) ALANINE (UNII: OF5P57N2ZX) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 09/27/2016 Part 2 of 2 SALINE DILUENT
sodium chloride diluent injectionProduct Information Item Code (Source) NDC: 49281-911 Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-911-58 0.5 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 09/27/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 09/27/2016 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur SA 578763542 MANUFACTURE(49281-913)
Trademark Results [STAMARIL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STAMARIL 79072978 3814773 Dead/Cancelled |
INSTITUT PASTEUR 2009-08-06 |
 STAMARIL 76503347 2885789 Dead/Cancelled |
Institut Pasteur 2003-04-03 |
 STAMARIL 76284886 not registered Dead/Abandoned |
Institut Pasteur 2001-07-13 |
 STAMARIL 73755315 1543214 Dead/Cancelled |
INSTITUT PASTEUR 1988-10-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.