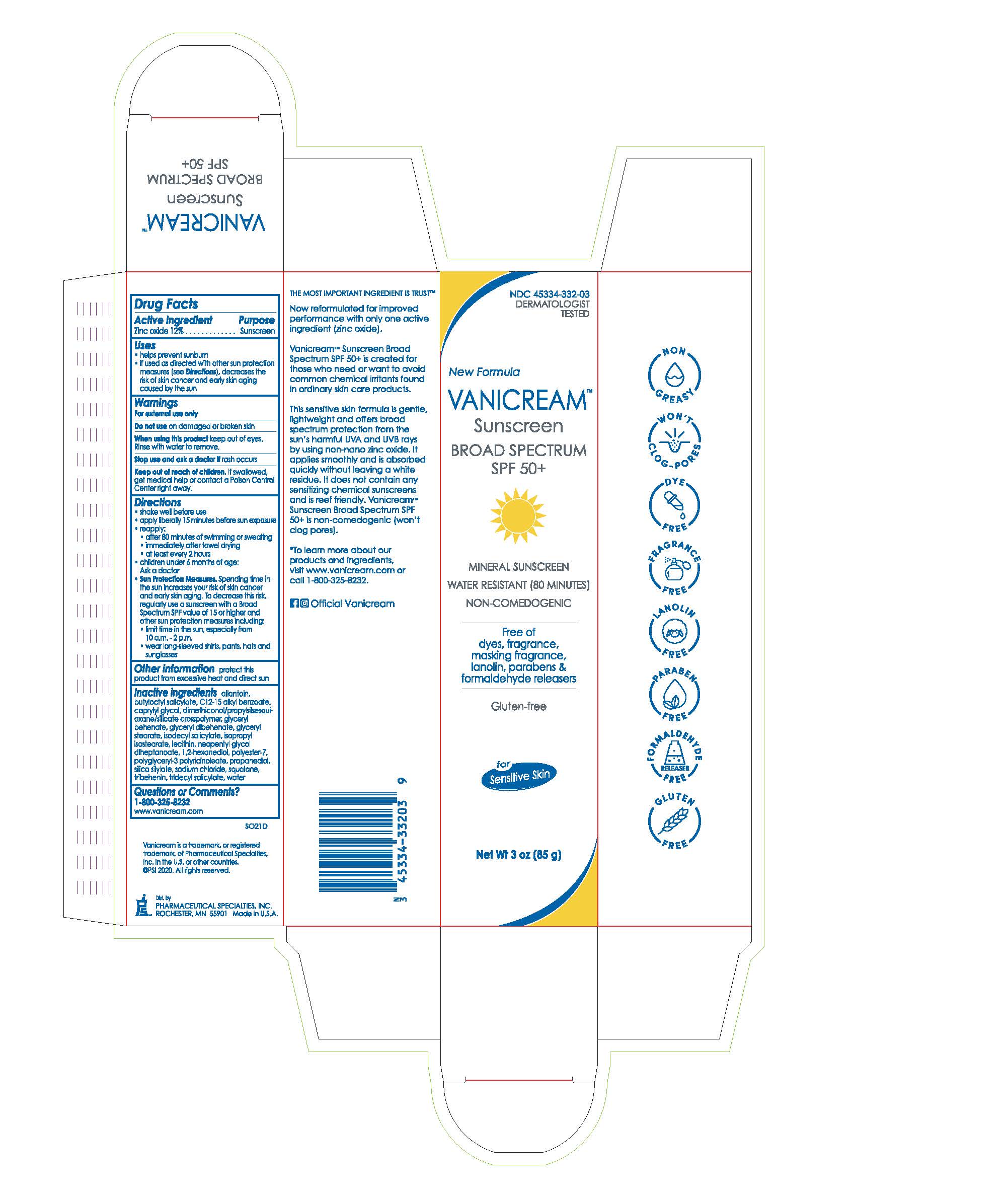
VANICREAM Sunscreen BROAD SPECTRUM SPF 50+
Vanicream Sunscreen Broad Spectrum SPF 50 by
Drug Labeling and Warnings
Vanicream Sunscreen Broad Spectrum SPF 50 by is a Otc medication manufactured, distributed, or labeled by Pharmaceutical Specialties, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VANICREAM SUNSCREEN BROAD SPECTRUM SPF 50- zinc oxide cream
Pharmaceutical Specialties, Inc.
----------
VANICREAM Sunscreen BROAD SPECTRUM SPF 50+
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- shake well before use
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. -2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
Inactive ingredients allantoin, butyloctyl salicylate, C12-15 alkyl benzoate, caprylyl glycol, dimethiconol/propylsilsesquioxane/silicate crosspolymer, glyceryl behenate, glyceryl dibehenate, glyceryl stearate, isodecyl salicylate, isopropyl isostearate, lecithin, neopentyl glycol diheptanoate, 1,2-hexanediol, polyester-7, polyglyceryl-3 polyricinoleate, propanediol, silica silylate, sodium chloride, squalane, tribehenin, tridecyl salicylate, water
Vanicream is a trademark, or registered trademark of Pharmaceutical Specialties, Inc. in the U.S. or other countries. ©PSI 2020. All rights reserved.
Dist. by
PHARMACEUTICAL SPECIALTIES, INC.
ROCHESTER, MN 55901 Made in USA
NDC: 45334-332-03
DERMATOLOGIST TESTED
New Formula
VANICREAM™ Sunscreen
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
WATER RESISTANT (80 MINUTES)
NON-COMEDOGENIC
Free of dyes, fragrance, masking fragrance, lanolin, parabens, and formaldehyde releasers
Gluten-Free
for Sensitive Skin
Net Wt 3 oz (85 g)
| VANICREAM SUNSCREEN BROAD SPECTRUM SPF 50
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Pharmaceutical Specialties, Inc. (076499557) |