BRINZOLAMIDE suspension/ drops
BRINZOLAMIDE by
Drug Labeling and Warnings
BRINZOLAMIDE by is a Prescription medication manufactured, distributed, or labeled by Padagis US LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BRINZOLAMIDE OPHTHALMIC SUSPENSION safely and effectively. See full prescribing information for BRINZOLAMIDE OPHTHALMIC SUSPENSION.
BRINZOLAMIDE ophthalmic suspension 1%, for topical ophthalmic use
Initial U.S. Approval: 1998RECENT MAJOR CHANGES
Warnings and Precautions,
Sulfonamide Hypersensitivity Reactions (5.1) 6/2023INDICATIONS AND USAGE
Brinzolamide ophthalmic suspension is a carbonic anhydrase inhibitor indicated for the treatment of elevated intraocular pressure (IOP) in patients with ocular hypertension or open-angle glaucoma. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Ophthalmic suspension containing brinzolamide 1% (10 mg/mL). (3)
CONTRAINDICATIONS
- Hypersensitivity to any component of this product. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence 5% to 10%) are blurred vision and bitter, sour, or unusual taste. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sulfonamide Hypersensitivity Reactions
5.2 Corneal Endothelium
5.3 Severe Renal Impairment
5.4 Acute Angle-Closure Glaucoma
5.5 Risk of Contamination
5.6 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Oral Carbonic Anhydrase Inhibitors
7.2 High-Dose Salicylate Therapy
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dose is one drop of brinzolamide ophthalmic suspension in the affected eye(s) 3 times daily. Shake well before use. Brinzolamide ophthalmic suspension may be used concomitantly with other topical ophthalmic drug products to lower IOP. If more than one topical ophthalmic drug is being used, the drugs should be administered at least 10 minutes apart.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Sulfonamide Hypersensitivity Reactions
Brinzolamide ophthalmic suspension is a sulfonamide and although administered topically, it is absorbed systemically. Therefore, the same types of adverse reactions that are attributable to sulfonamides may occur with topical administration of brinzolamide ophthalmic suspension. Fatalities have occurred, although rarely, due to severe reactions to sulfonamides, including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Sensitization may recur when a sulfonamide is readministered irrespective of the route of administration. If signs of serious reactions or hypersensitivity occur, discontinue the use of this preparation immediately.
5.2 Corneal Endothelium
Carbonic anhydrase activity has been observed in both the cytoplasm and around the plasma membranes of the corneal endothelium. There is an increased potential for developing corneal edema in patients with low endothelial cell counts. Caution should be used when prescribing brinzolamide ophthalmic suspension to this group of patients.
5.3 Severe Renal Impairment
Brinzolamide ophthalmic suspension has not been studied in patients with severe renal impairment [creatinine clearance (CrCl) less than 30 mL/min]. Because brinzolamide ophthalmic suspension and its metabolite are excreted predominantly by the kidney, Brinzolamide ophthalmic suspension is not recommended in such patients.
5.4 Acute Angle-Closure Glaucoma
The management of patients with acute angle-closure glaucoma requires therapeutic interventions in addition to ocular hypotensive agents. Brinzolamide ophthalmic suspension has not been studied in patients with acute angle-closure glaucoma.
5.5 Risk of Contamination
Avoid allowing the tip of the dispensing container to contact the eye or surrounding structures or other surfaces, since the product can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies of brinzolamide ophthalmic suspension, the most frequently reported adverse reactions reported in 5% to 10% of patients were blurred vision and bitter, sour, or unusual taste. Adverse reactions occurring in 1% to 5% of patients were blepharitis, dermatitis, dry eye, foreign body sensation, headache, hyperemia, ocular discharge, ocular discomfort, ocular keratitis, ocular pain, ocular pruritus, and rhinitis.
The following adverse reactions were reported at an incidence below 1%: allergic reactions, alopecia, chest pain, conjunctivitis, diarrhea, diplopia, dizziness, dry mouth, dyspnea, dyspepsia, eye fatigue, hypertonia, keratoconjunctivitis, keratopathy, kidney pain, lid margin crusting or sticky sensation, nausea, pharyngitis, tearing, and urticaria.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of brinzolamide containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious skin and subcutaneous tissue reactions such as Stevens-Johnson syndrome (SJS) and Toxic epidermal necrolysis (TEN) may occur with the use of brinzolamide due to its sulfonamide component [see Warnings and Precautions (5.1)].
-
7 DRUG INTERACTIONS
7.1 Oral Carbonic Anhydrase Inhibitors
There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and brinzolamide ophthalmic suspension. The concomitant administration of brinzolamide ophthalmic suspension and oral carbonic anhydrase inhibitors is not recommended.
7.2 High-Dose Salicylate Therapy
Carbonic anhydrase inhibitors may produce acid-base and electrolyte alterations. These alterations were not reported in the clinical trials with brinzolamide. However, in patients treated with oral carbonic anhydrase inhibitors, rare instances of acid-base alterations have occurred with high-dose salicylate therapy. Therefore, the potential for such drug interactions should be considered in patients receiving brinzolamide ophthalmic suspension.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women to inform drug-associated risk.
In reproductive toxicity studies, brinzolamide administered orally to rats induced fetal toxicity at 375 times the recommended human ophthalmic dose (RHOD) based on mg/kg. In rabbits, no fetal toxicity was observed following oral administration (see Data). The background risk of major birth defects and miscarriage for the indicated population is unknown; however, in the U.S. general population, the estimated background risk of major birth defects is 2% to 4%, and of miscarriage is 15% to 20%, of clinically recognized pregnancies.
Data
Animal Data
Embryo-fetal studies were conducted in pregnant rats administered 0, 2, 6, or 18 mg/kg/day brinzolamide by oral gavage on gestation days 6 to 17, to target the period of organogenesis. Decreased fetal body weight with reduced skeletal ossification were observed at 18 mg/kg/day (375 times the RHOD based on mg/kg). The no-observed-adverse-effect-level (NOAEL) for fetal toxicity was 6 mg/kg/day (125 times the RHOD). Decreased maternal weight gain was observed at 18 mg/kg/day. The NOAEL for maternal toxicity was 6 mg/kg/day (125 times the RHOD). Embryo-fetal studies were conducted in pregnant rabbits administered 0, 1, 3, or 6 mg/kg/day of brinzolamide by oral gavage on gestation days 6 to 18, to target the period of organogenesis. No treatment-related fetal effects were observed at any dose. The NOAEL for fetal toxicity was 6 mg/kg/day (125 times the RHOD based on mg/kg). Maternal weight loss during pregnancy was observed at 3 mg/kg/day (63 times the RHOD) and above. The NOAEL for maternal toxicity was 1 mg/kg/day (21 times the RHOD).
A peri-/postnatal study was conducted in rats administered brinzolamide by oral gavage from gestation day 16 through lactation day 20. Decreased pup body weight was observed at 15 mg/kg/day (313 times the RHOD based on mg/kg). The NOAEL for developmental toxicity was 5 mg/kg/day (104 times the RHOD). Following oral administration of 14C-brinzolamide to pregnant rats, radioactivity was found to cross the placenta and was present in the fetal tissues and blood.
8.2 Lactation
Risk Summary
There are no data on the presence of brinzolamide in human milk, the effects on the breastfed infant, or the effects on milk production. Brinzolamide has been detected in the milk of lactating rats.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for brinzolamide ophthalmic suspension and any potential adverse effects on the breastfed child from brinzolamide ophthalmic suspension.
8.4 Pediatric Use
A 3-month controlled clinical study was conducted in which brinzolamide ophthalmic suspension was dosed only twice a day in pediatric patients 4 weeks to 5 years of age. Patients were not required to discontinue their IOP-lowering medication(s) until initiation of monotherapy with brinzolamide ophthalmic suspension. IOP-lowering efficacy was not demonstrated in this study in which the mean decrease in elevated IOP was between 0 mmHg and 2 mmHg. Five out of 32 patients demonstrated an increase in corneal diameter of one millimeter.
- 10 OVERDOSAGE
-
11 DESCRIPTION
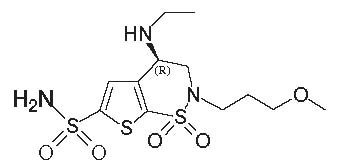
Brinzolamide ophthalmic suspension, USP 1% contains a carbonic anhydrase inhibitor formulated for multidose topical ophthalmic use. Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1,2-thiazine-6-sulfonamide-1,1- dioxide. Its empirical formula is C12H21N3O5S3, and its structural formula is:
Brinzolamide has a molecular weight of 383.5 g/mol and a melting point of about 131°C. It is a white powder, which is insoluble in water, very soluble in methanol and soluble in ethanol.
Brinzolamide ophthalmic suspension, USP 1% is supplied as a sterile, aqueous suspension of brinzolamide which has been formulated to be readily suspended and slow settling, following shaking. It has a pH of approximately 7.5 and an osmolality of 300 mOsm/kg.
Each mL of brinzolamide ophthalmic suspension, USP 1% contains: Active ingredient: brinzolamide 10 mg; Preservative: Benzalkonium chloride 0.1 mg; Inactives: carbomer 974P, edetate disodium dihydrate, with hydrochloric acid and/or sodium hydroxide to adjust pH, mannitol, sodium chloride, tyloxapol, and water for injection. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Carbonic anhydrase is an enzyme found in many tissues of the body, including the eye. It catalyzes the reversible reaction involving the hydration of carbon dioxide and the dehydration of carbonic acid. In humans, carbonic anhydrase exists as a number of isoenzymes, the most active being carbonic anhydrase II, found primarily in red blood cells (RBCs), but also in other tissues. Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport. The result is a reduction in IOP.
Brinzolamide ophthalmic suspension contains brinzolamide, an inhibitor of carbonic anhydrase II. Following topical ocular administration, brinzolamide inhibits aqueous humor formation and reduces elevated IOP. Elevated IOP is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss.12.3 Pharmacokinetics
Following topical ocular administration, brinzolamide is absorbed into the systemic circulation. Due to its affinity for carbonic anhydrase II, brinzolamide distributes extensively into the RBCs and exhibits a long half-life in whole blood (approximately 111 days). In humans, the metabolite N-desethyl brinzolamide is formed, which also binds to carbonic anhydrase and accumulates in RBCs. This metabolite binds mainly to carbonic anhydrase I in the presence of brinzolamide. In plasma, both parent brinzolamide and N-desethyl brinzolamide concentrations are low and generally below assay quantitation limits (less than 10 ng/mL). Binding to plasma proteins is approximately 60%. Brinzolamide is eliminated predominantly in the urine as unchanged drug. N-Desethyl brinzolamide is also found in the urine along with lower concentrations of the N-desmethoxypropyl and O-desmethyl metabolites.
An oral pharmacokinetic study was conducted in which healthy volunteers received 1 mg capsules of brinzolamide twice per day for up to 32 weeks. This regimen approximates the amount of drug delivered by topical ocular administration of brinzolamide ophthalmic suspension dosed to both eyes 3 times per day and simulates systemic drug and metabolite concentrations similar to those achieved with long-term topical dosing. Red blood cell carbonic anhydrase activity was measured to assess the degree of systemic carbonic anhydrase inhibition. Brinzolamide saturation of RBC carbonic anhydrase II was achieved within 4 weeks (RBC concentrations of approximately 20 mcM). N-Desethyl brinzolamide accumulated in RBCs to steady-state within 20 to 28 weeks reaching concentrations ranging from 6 to 30 mcM. The inhibition of carbonic anhydrase II activity at steady-state was approximately 70% to 75%, which is below the degree of inhibition expected to have a pharmacological effect on renal function or respiration in healthy subjects. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Brinzolamide caused urinary bladder tumors in female mice at oral doses of 10 mg/kg/day and in male rats at oral doses of 8 mg/kg/day in 2-year studies. Brinzolamide was not carcinogenic in male mice or female rats dosed orally for up to 2 years. The carcinogenicity appears secondary to kidney and urinary bladder toxicity. These levels of exposure cannot be achieved with topical ophthalmic dosing in humans.
Mutagenesis
The following tests for mutagenic potential were negative: (1) in vivo mouse micronucleus assay; (2) in vivo sister chromatid exchange assay; and (3) Ames E. coli test. The in vitro mouse lymphoma forward mutation assay was negative in the absence of activation, but positive in the presence of microsomal activation.
Impairment of Fertility
In reproduction studies of brinzolamide in rats, there were no adverse effects on the fertility or reproductive capacity of males or females at doses up to 18 mg/kg/day (375 times the RHOD based on mg/kg).
-
14 CLINICAL STUDIES
In two, 3-month clinical studies, brinzolamide ophthalmic suspension dosed 3 times per day in patients with elevated IOP, produced significant reductions in IOPs (4 mmHg to 5 mmHg). These IOP reductions are equivalent to the reductions observed with dorzolamide hydrochloride ophthalmic solution 2% dosed 3 times per day in the same studies.
In 2 clinical studies in patients with elevated IOP, brinzolamide ophthalmic suspension was associated with less stinging and burning upon instillation than dorzolamide hydrochloride ophthalmic solution 2%. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Brinzolamide ophthalmic suspension, USP 1% is supplied in a plastic bottle with a controlled drip tip and an orange cap.
10 mL NDC: 0574-4012-10
15 mL NDC: 0574-4012-15
Storage and Handling
Store brinzolamide ophthalmic suspension at 4°C to 30°C (39°F to 86°F). Shake well before use. After opening, brinzolamide ophthalmic suspension can be used until the expiration date on the bottle. -
17 PATIENT COUNSELING INFORMATION
Sulfonamide Hypersensitivity Reactions
Advise patients that if serious or unusual ocular or systemic reactions or signs of hypersensitivity occur, they should discontinue the use of the product and consult their physician immediately [see Warnings and Precautions (5.1)].
Temporary Blurred Vision
Vision may be temporarily blurred following dosing with brinzolamide ophthalmic suspension. Advise patients to exercise care in operating machinery or driving a motor vehicle.
Avoiding Contamination of the Product
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures or other surfaces, since the product can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Intercurrent Ocular Conditions
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multi-dose container.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic drug is being used, the drugs should be administered at least ten minutes apart.
Contact Lens Wear
The preservative in brinzolamide ophthalmic suspension, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of brinzolamide ophthalmic suspension, but may be reinserted 15 minutes after instillation.
Manufactured for Padagis®
Minneapolis, MN 55427www.padagis.com
Rev 08-24
3Y000 RC PH5
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRINZOLAMIDE
brinzolamide suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0574-4012 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRINZOLAMIDE (UNII: 9451Z89515) (BRINZOLAMIDE - UNII:9451Z89515) BRINZOLAMIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.1 mg in 1 mL CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) EDETATE DISODIUM (UNII: 7FLD91C86K) MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) TYLOXAPOL (UNII: Y27PUL9H56) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0574-4012-10 1 in 1 CARTON 10/10/2023 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0574-4012-15 1 in 1 CARTON 10/10/2023 2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211914 10/10/2023 Labeler - Padagis US LLC (967694121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.