PainBloc by Denison Pharmaceuticals, LLC PainBloc
PainBloc by
Drug Labeling and Warnings
PainBloc by is a Otc medication manufactured, distributed, or labeled by Denison Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
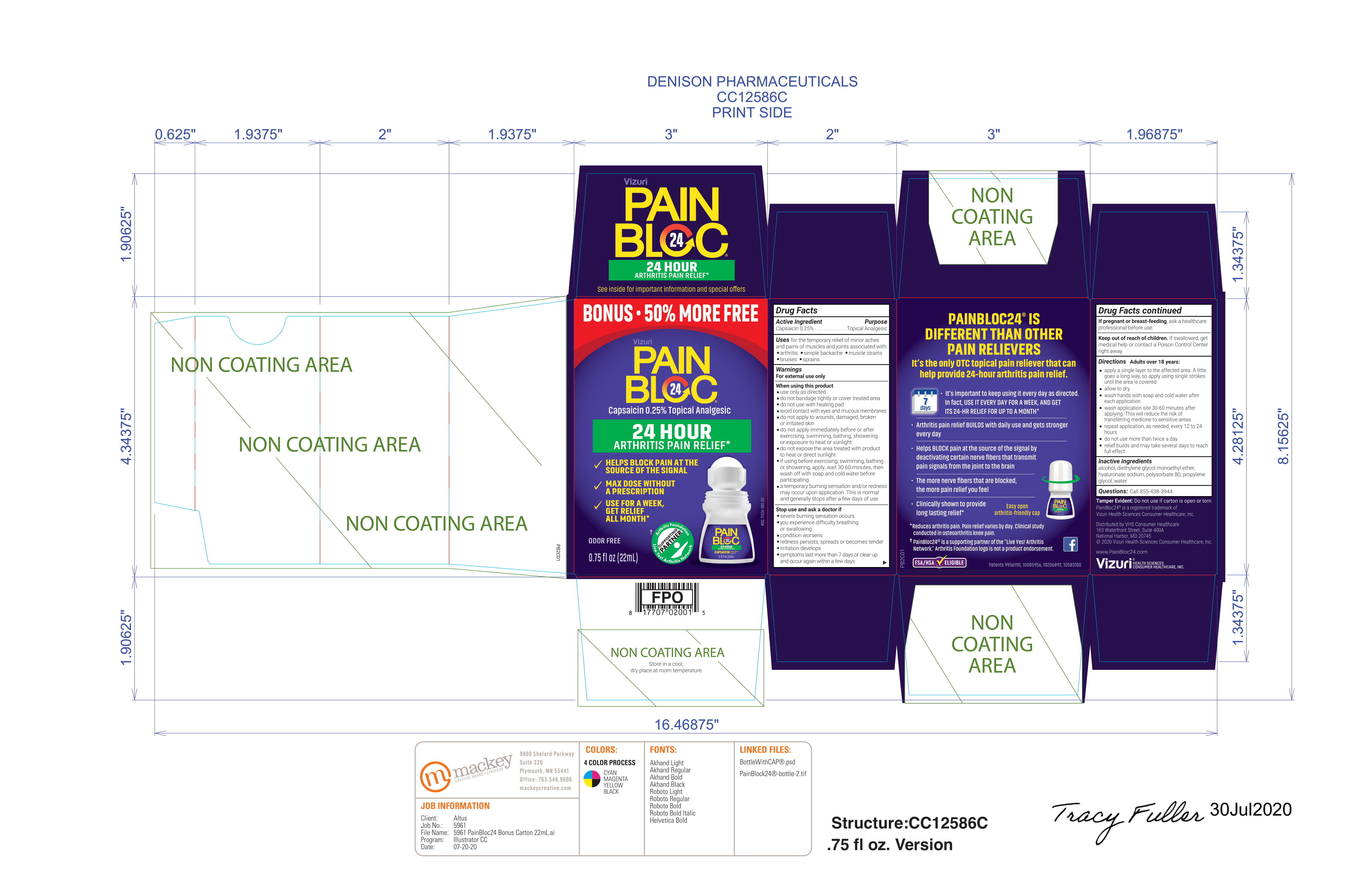
PAINBLOC- capsaicin 0.25% liquid
Denison Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PainBloc
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with:
- Arthritis
- Simple backache
- Muscle strains
- Bruises
- Sprains
Warnings
For external use only
When using this product
- Use only as directed
- Do not bandage tightly or cover treated area
- Do not use with heating pad
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds, damaged, broken or irritated skin
- Do not apply immediately before or after exercising, swimming, bathing, showering or exposure to heat or sunlight
- Do not expose the area treated with product to heat or direct sunlight
- If using before exercise, showering or swimming, apply, wait 30-60 minutes, then wash off with soap and cold water before participating
- A temporary burning sensation and/or redness may occur upon application. This is normal and generally stops after a few days of use
Directions
Adults over 18 years:
- Apply a single layer to the affected area. A little goes a long way, so apply using single strokes until the area is covered.
- Allow to dry
- Wash hands with soap and cold water after each application
- Wash application site 30-60 minutes after applying. This will reduce the risk of transferring medicine to sensitive areas
- repeat application as needed, every 12 to 24 hours
- Do not use more than twice a day
- Relief builds and may take several days to reach full effect
| PAINBLOC
capsaicin 0.25% liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, LLC (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, LLC | 001207208 | manufacture(0295-9034) | |
Revised: 12/2020
Document Id: b57d0e22-2c2e-099d-e053-2a95a90ab309
Set id: b57d0cfc-1592-c14a-e053-2995a90a6286
Version: 1
Effective Time: 20201202
Trademark Results [PainBloc]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PAINBLOC 87220812 not registered Dead/Abandoned |
Vizuri Health Sciences LLC 2016-10-31 |
 PAINBLOC 75304587 not registered Dead/Abandoned |
Enamelon, Inc. 1997-06-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.