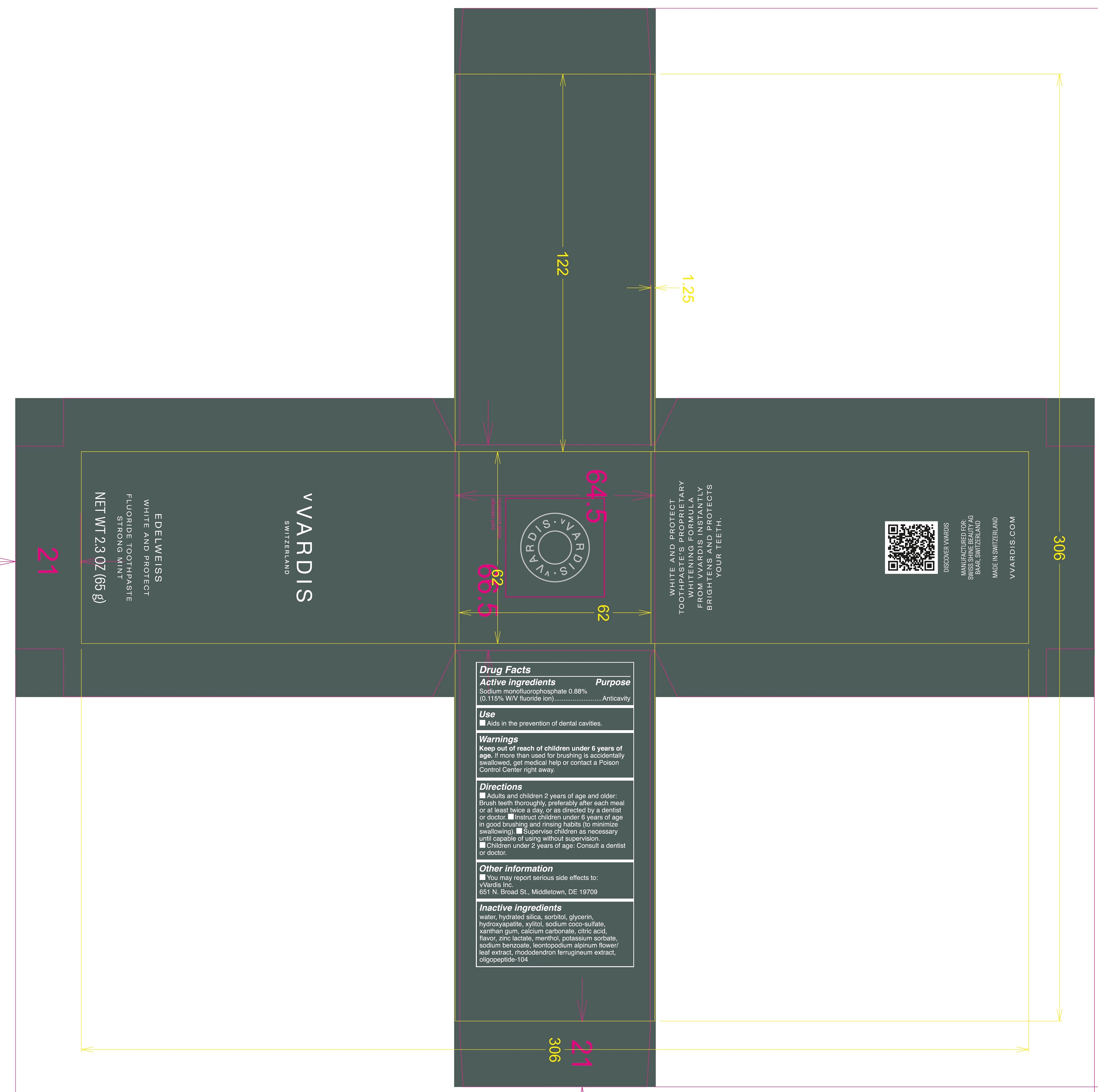
vVARDIS Switzerland Edelweiss White and Protect Fluoride Toothpaste Strong Mint
vVARDIS Switzerland Edelweiss White and Protect Fluoride Tooth Strong Mint by
Drug Labeling and Warnings
vVARDIS Switzerland Edelweiss White and Protect Fluoride Tooth Strong Mint by is a Otc medication manufactured, distributed, or labeled by Marvinpac SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VVARDIS SWITZERLAND EDELWEISS WHITE AND PROTECT FLUORIDE TOOTH STRONG MINT- sodium monofluorophosphate paste
Marvinpac SA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
vVARDIS Switzerland Edelweiss White and Protect Fluoride Toothpaste Strong Mint
Directions
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing),
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or a doctor.
Other information
- You may report serious side effects to: vVardis Inc.
651 N. Broad St., Middletown, DE 19709
Inactive ingredients
water, hydrated silica, sorbitol, glycerin, hydroxyyapatite, xylitol, sodium coco-sulfate, xanthan gum, calcium carbonate, citric acid, flavor, zinc lactate, menthol, potassium sorbate, sodium benzoate, leontopodium alpinum flower/leaf extract, rhododendron ferruginem extract, oligopeptide-104
| VVARDIS SWITZERLAND EDELWEISS WHITE AND PROTECT FLUORIDE TOOTH STRONG MINT
sodium monofluorophosphate paste |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Marvinpac SA (482060329) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.