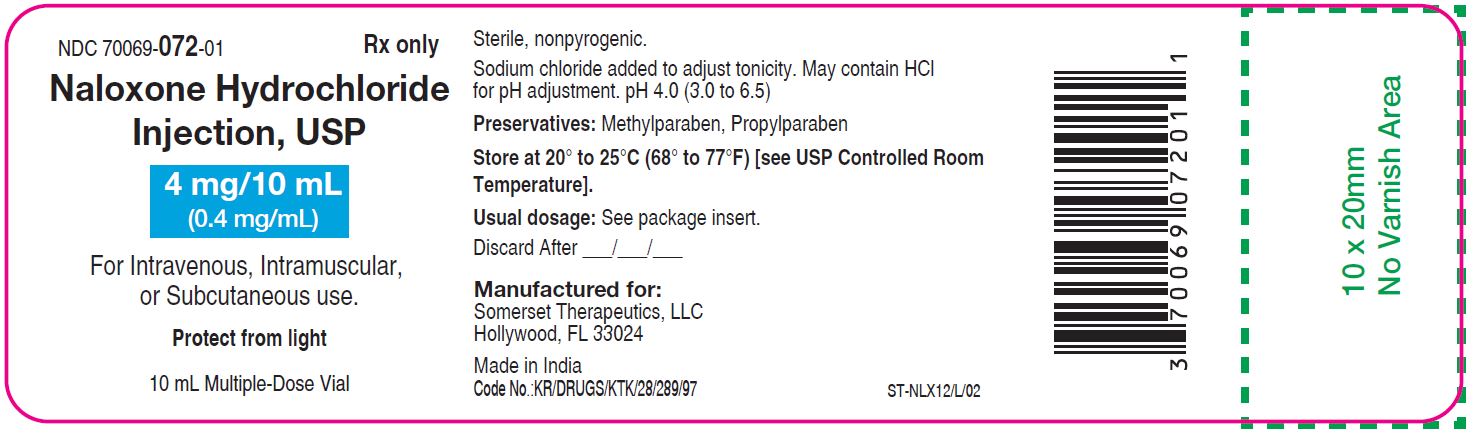
NALOXONE HYDROCHLORIDE injection, solution
Naloxone Hydrochloride by
Drug Labeling and Warnings
Naloxone Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Somerset Therapeutics, LLC, Wintac Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Naloxone Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of naloxone hydrochloride in water for injection. Each milliliter (mL) contains 0.4 mg naloxone hydrochloride and sodium chloride to adjust tonicity in water for injection. May contain hydrochloric acid for pH adjustment; pH 4.0 (3.0 to 6.5).
The multiple-dose solution contains, in addition, 1.8 mg/mL methylparaben and 0.2 mg/mL propylparaben added as preservatives.
Naloxone Hydrochloride Injection, USP may be administered intravenously, intramuscularly, or subcutaneously.
Naloxone, an opioid antagonist, is a synthetic congener of oxymorphone. It differs from oxymorphone in that the methyl group on the nitrogen atom is replaced by an allyl group.
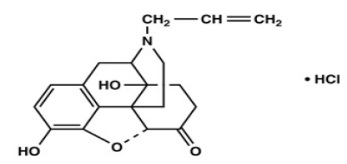
Naloxone Hydrochloride, USP is chemically designated 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride (C19H21NO4 HCl), a white to slightly off-white powder soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and chloroform. It has a molecular weight of 363.84. It has the following structural formula:
-
CLINICAL PHARMACOLOGY
Complete or Partial Reversal of Opioid Depression
Naloxone prevents or reverses the effects of opioids including respiratory depression, sedation and hypotension. Also, Naloxone can reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
Naloxone is an essentially pure opioid antagonist, i.e., it does not possess the "agonistic" or morphine-like properties characteristic of other opioid antagonists. When administered in usual doses and in the absence of opioids or agonistic effects of other opioid antagonists, it exhibits essentially no pharmacologic activity.
Naloxone has not been shown to produce tolerance or cause physical or psychological dependence. In the presence of physical dependence on opioids, naloxone will produce withdrawal symptoms. However, in the presence of opioid dependence, opiate withdrawal symptoms may appear within minutes of naloxone administration and will subside in about 2 hours. The severity and duration of the withdrawal syndrome are related to the dose of naloxone and to the degree and type of opioid dependence.
While the mechanism of action of naloxone is not fully understood, in vitro evidence suggests that naloxone antagonizes opioid effects by competing for the mu, kappa, and sigma opiate receptor sites in the CNS, with the greatest affinity for the mu receptor.
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes; the onset of action is slightly less rapid when it is administered subcutaneously or intramuscularly. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride. Intramuscular administration produces a more prolonged effect than intravenous administration. Since the duration of action of naloxone may be shorter than that of some opiates, the effects of the opiate may return as the effects of naloxone dissipates.
The requirement for repeat doses of naloxone, however, will also be dependent upon the amount, type and route of administration of the opioid being antagonized.
Adjunctive Use in Septic Shock
Naloxone has been shown in some cases of septic shock to produce a rise in blood pressure that may last up to several hours; however this pressor response has not been demonstrated to improve patient survival. In some studies, treatment with naloxone in the setting of septic shock has been associated with adverse effects, including agitation, nausea and vomiting, pulmonary edema, hypotension, cardiac arrhythmias, and seizures. The decision to use naloxone in septic shock should be exercised with caution, particularly in patients who may have underlying pain or have previously received opioid therapy and may have developed opioid tolerance.
Because of the limited number of patients who have been treated, optimal dosage and treatment regimens have not been established.
-
PHARMACOKINETICS
Distribution
Following parenteral administration, naloxone is rapidly distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent but significant binding of naloxone also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.
Metabolism and Elimination
Naloxone is metabolized in the liver primarily by glucuronide conjugation with naloxone-3-glucuronide as the major metabolite. In one study, the serum half-life in adults ranged from 30 to 81 minutes (mean 64 ± 12 minutes). In a neonatal study, the mean plasma half-life was observed to be 3.1 ± 0.5 hours. After an oral or intravenous dose, about 25 to 40% of the drug is excreted as metabolites in urine within 6 hours, about 50% in 24 hours, and 60 to 70% in 72 hours.
-
INDICATIONS AND USAGE
Naloxone Hydrochloride Injection is indicated for the complete or partial reversal of opioid depression, including respiratory depression, induced by natural and synthetic opioids including propoxyphene, methadone, and certain mixed agonist-antagonist analgesics: nalbuphine, pentazocine, butorphanol, and cyclazocine. Naloxone hydrochloride is also indicated for the diagnosis of suspected or known acute opioid overdosage.
Naloxone may be useful as an adjunctive agent to increase blood pressure in the management of septic shock (see CLINICAL PHARMACOLOGY; Adjunctive Use in Septic Shock).
- CONTRAINDICATIONS
-
WARNINGS
Drug Dependence
Naloxone hydrochloride injection should be administered cautiously to persons, including newborns of mothers, who are known or suspected to be physically dependent on opioids. In such cases an abrupt and complete reversal of opioid effects may precipitate an acute withdrawal syndrome.
The signs and symptoms of opioid withdrawal in a patient physically dependent on opioids may include, but are not limited to, the following: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In the neonate, opioid withdrawal may also include: convulsions, excessive crying, and hyperactive reflexes.
Repeat Administration
The patient who has satisfactorily responded to naloxone should be kept under continued surveillance and repeated doses of naloxone should be administered, as necessary, since the duration of action of some opioids may exceed that of naloxone.
Respiratory Depression Due to Other Drugs
Naloxone is not effective against respiratory depression due to non-opioid drugs and in the management of acute toxicity caused by levopropoxyphene. Reversal of respiratory depression by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete or require higher doses of naloxone. If an incomplete response occurs, respirations should be mechanically assisted as clinically indicated.
-
PRECAUTIONS
General
In addition to naloxone, other resuscitative measures such as maintenance of a free airway, artificial ventilation, cardiac massage, and vasopressor agents should be available and employed when necessary to counteract acute opioid poisoning.
Abrupt postoperative reversal of opioid depression may result in nausea, vomiting, sweating, tremulousness, tachycardia, increased blood pressure, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest which may result in death. Excessive doses of naloxone in postoperative patients may result in significant reversal of analgesia and may cause agitation (see PRECAUTIONS and DOSAGE AND ADMINISTRATION; Usage in Adults, Postoperative Opioid Depression).
Several instances of hypotension, hypertension, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest have been reported in postoperative patients. Death, coma, and encephalopathy have been reported as sequelae of these events. These have occurred in patients most of whom had pre-existing cardiovascular disorders or received other drugs which may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, naloxone should be used with caution in patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects such as hypotension, ventricular tachycardia or fibrillation and pulmonary edema. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
Drug Interactions
Large doses of naloxone are required to antagonize buprenorphine since the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression. The barbiturate methohexital appears to block the acute onset of withdrawal symptoms induced by naloxone in opiate addicts.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals to assess the carcinogenic potential of naloxone have not been conducted. Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study. Reproduction studies conducted in mice and rats at doses 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg/day (when based on surface area or mg/m2), demonstrated no embryotoxic or teratogenic effects due to naloxone.
Use in Pregnancy: Teratogenic Effects: Pregnancy Category C
Teratology studies conducted in mice and rats at doses 4-times and 8- times, respectively, the dose of a 50 kg human given 10 mg/day (when based on surface area or mg/m2), demonstrated no embryotoxic or teratogenic effects due to naloxone. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, naloxone hydrochloride should be used during pregnancy only if clearly needed.
Non-teratogenic effects: Risk-benefit must be considered before naloxone is administered to a pregnant woman who is known or suspected to be opioid-dependent since maternal dependence may often be accompanied by fetal dependence. Naloxone crosses the placenta, and may precipitate withdrawal in the fetus as well as in the mother. Patients with mild to moderate hypertension who receive naloxone during labor should be carefully monitored as severe hypertension may occur.
Use in Labor and Delivery
It is not known if naloxone hydrochloride injection affects the duration of labor and/or delivery. However, published reports indicated that the administration of naloxone during labor did not adversely affect maternal or neonatal status.
Nursing Mothers
It is not known whether naloxone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when naloxone hydrochloride is administered to a nursing woman.
Pediatric Use
Naloxone hydrochloride injection may be administered intravenously, intramuscularly, or subcutaneously in children and neonates to reverse the effects of opiates. The American Academy of Pediatrics, however, does not endorse subcutaneous or intramuscular administration in opiate intoxication since absorption may be erratic or delayed. Although the opiate-intoxicated child responds dramatically to naloxone hydrochloride injection, he/she must be carefully monitored for at least 24 hours as a relapse may occur as naloxone is metabolized.
When naloxone hydrochloride injection is given to the mother shortly before delivery, the duration of its effects lasts only for the first two hours of neonatal life. It is preferable to administer naloxone hydrochloride injection directly to the neonate if needed after delivery. Naloxone has no apparent benefit as an additional method of resuscitation in the newly born infant with intrauterine asphyxia, which is not related to opioid use.
Usage in Pediatric Patients and Neonates for Septic Shock: The safety and effectiveness of naloxone hydrochloride injection in the treatment of hypotension in pediatric patients and neonates with septic shock have not been established. One study of two neonates in septic shock reported a positive pressor response; however, one patient subsequently died after intractable seizures.
Geriatric Use
Clinical studies of naloxone hydrochloride injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Postoperative
The following adverse events have been associated with the use of naloxone hydrochloride injection in postoperative patients: hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone in postoperative patients may result in significant reversal of analgesia and may cause agitation (see PRECAUTIONS and DOSAGE AND ADMINISTRATION; Usage in Adults, Postoperative Opioid Depression).
Opioid Depression
Abrupt reversal of opioid depression may result in nausea, vomiting, sweating, tachycardia, increased blood pressure, tremulousness, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest which may result in death (see PRECAUTIONS).
Opioid Dependence
Abrupt reversal of opioid effects in persons who are physically dependent on opioids may precipitate an acute withdrawal syndrome which may include, but is not limited to the following signs and symptoms: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, and tachycardia. In the neonate, opioid withdrawal may also include: convulsions, excessive crying, and hyperactive reflexes (See WARNINGS).
Adverse events associated with the postoperative use of naloxone hydrochloride injection are listed by organ system and in decreasing order of frequency as follows:
Cardiac Disorders: pulmonary edema, cardiac arrest or failure, tachycardia, ventricular fibrillation, and ventricular tachycardia. Death, coma, and encephalopathy have been reported as sequelae of these events.
Gastrointestinal Disorders: vomiting, nausea
Nervous System Disorders: convulsions, paraesthesia, grand mal convulsion
Psychiatric Disorders: agitation, hallucination, tremulousness
Respiratory, Thoracic, and Mediastinal Disorders: dyspnea, respiratory depression, hypoxia
Skin and Subcutaneous Tissue Disorders: nonspecific injection site reactions, sweating
Vascular Disorders: hypertension, hypotension, hot flashes or flushing
See also PRECAUTIONS and DOSAGE AND ADMINISTRATION; Usage in Adults, Postoperative Opioid Depression.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
There is limited clinical experience with naloxone hydrochloride injection overdosage in humans.
Adult Patients
In one small study, volunteers who received 24 mg/70 kg did not demonstrate toxicity.
In another study, 36 patients with acute stroke received a loading dose of 4 mg/kg (10 mg/m2/min) of naloxone hydrochloride injection followed immediately by 2 mg/kg/hr for 24 hours. Twenty-three patients experienced adverse events associated with naloxone use, and naloxone was discontinued in seven patients because of adverse effects. The most serious adverse events were: seizures (2 patients), severe hypertension (1), and hypotension and/or bradycardia (3).
At doses of 2 mg/kg in normal subjects, cognitive impairment and behavioral symptoms, including irritability, anxiety, tension, suspiciousness, sadness, difficulty concentrating, and lack of appetite have been reported. In addition, somatic symptoms, including dizziness, heaviness, sweating, nausea, and stomachaches were also reported. Although complete information is not available, behavioral symptoms were reported to often persist for 2 to 3 days.
Pediatric Patients
Up to 11 doses of 0.2 mg naloxone (2.2 mg) have been administered to children following overdose of diphenoxylate hydrochloride with atropine sulfate. Pediatric reports include a 2½ year-old child who inadvertently received a dose of 20 mg naloxone for treatment of respiratory depression following overdose with diphenoxylate hydrochloride with atropine sulfate. The child responded well and recovered without adverse sequelae. There is also a report of a 4½ year-old child who received 11 doses during a 12-hour period, with no adverse sequelae.
-
DOSAGE AND ADMINISTRATION
Naloxone Hydrochloride Injection, may be administered intravenously, intramuscularly, or subcutaneously. The most rapid onset of action is achieved by intravenous administration and it is recommended in emergency situations.
Since the duration of action of some opioids may exceed that of naloxone, the patient should be kept under continued surveillance. Repeated doses of naloxone should be administered, as necessary.
Intravenous Infusion: Naloxone Hydrochloride Injection, may be diluted for intravenous infusion in 0.9% sodium chloride injection or 5% dextrose injection. The addition of 2 mg of naloxone hydrochloride in 500 mL of either solution provides a concentration of 0.004 mg/mL. Mixtures should be used within 24 hours. After 24 hours, the remaining unused solution must be discarded. The rate of administration should be titrated in accordance with the patient's response.
Naloxone Hydrochloride Injection, should not be mixed with preparations containing bisulfite, metabisulfite, long-chain or high molecular weight anions, or any solution having an alkaline pH. No drug or chemical agent should be added to Naloxone Hydrochloride Injection, unless its effect on the chemical and physical stability of the solution has first been established.
General
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not administer unless solution is clear and container is undamaged.
Opioid Overdose—Known or Suspected: An initial dose of 0.4 mg to 2 mg of naloxone hydrochloride may be administered intravenously. If the desired degree of counteraction and improvement in respiratory functions are not obtained, it may be repeated at two to three minute intervals. If no response is observed after 10 mg of naloxone hydrochloride have been administered, the diagnosis of opioid-induced or partial opioid-induced toxicity should be questioned. Intramuscular or subcutaneous administration may be necessary if the intravenous route is not available
Postoperative Opioid Depression: For the partial reversal of opioid depression following the use of opioids during surgery, smaller doses of naloxone hydrochloride are usually sufficient. The dose of naloxone should be titrated according to the patient's response. For the initial reversal of respiratory depression, naloxone hydrochloride should be injected in increments of 0.1 to 0.2 mg intravenously at two to three minute intervals to the desired degree of reversal, i.e., adequate ventilation and alertness without significant pain or discomfort. Larger than necessary dosage of naloxone may result in significant reversal of analgesia and increase in blood pressure. Similarly, too rapid reversal may induce nausea, vomiting, sweating or circulatory stress.
Repeat doses of naloxone may be required within one to two hour intervals depending upon the amount, type (i.e., short or long acting) and time interval since last administration of an opioid. Supplemental intramuscular doses have been shown to produce a longer lasting effect.
Septic Shock: The optimal dosage of Naloxone or duration of therapy for the treatment of hypotension in septic shock patients has not been established (see CLINICAL PHARMACOLOGY).
Usage in Pediatric Population:
Opioid Overdose – Known or Suspected: The usual initial dose in children is 0.01 mg/kg body weight given intravenously. If this dose does not result in the desired degree of clinical improvement, a subsequent dose of 0.1 mg/kg body weight may be administered. If an intravenous route of administration is not available, naloxone may be administered intramuscularly or subcutaneously in divided doses. If necessary, naloxone hydrochloride injection can be diluted with sterile water for injection.
Postoperative Opioid Depression: Follow the recommendations and cautions under Adult Postoperative Opioid Depression. For the initial reversal of respiratory depression naloxone hydrochloride should be injected in increments of 0.005 mg to 0.01 mg intravenously at two to three minute intervals to the desired degree of reversal.
Opioid-Induced Depression: The usual initial dose is 0.01 mg/kg body weight administered intravenously, intramuscularly or subcutaneously. This dose may be repeated in accordance with adult administration guidelines for postoperative opioid depression.
-
HOW SUPPLIED
Naloxone Hydrochloride Injection USP, 4 mg/10 mL (0.4 mg/mL) is a clear, colorless solution.
Naloxone Hydrochloride Injection USP, 4 mg/10 mL (0.4 mg/mL) for intravenous, intramuscular, and subcutaneous administration is available as:
NDC 70069-072-01 - 10 mL vials are Multiple-Dose Vials, packaged as monocarton.
NDC 70069-072-10 - 10 monocartons are packaged in an Inner Carton.
Store at 20° to 25°C (68° to 77°F). [see USP Controlled Room Temperature]. Protect from light.
Store in the carton until contents have been used.
Discard unused portion.
For Product Inquiry call 1-800-417-9175.
ST-NLX12/P/03
Revised: August, 2019
Manufactured for:
Somerset Therapeutics, LLC
Hollywood, FL 33024
Made in India
Code No.: KR/DRUGS/KTK/28/289/97
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70069-072 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 0.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.6 mg in 1 mL METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (Clear, Colorless) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70069-072-10 10 in 1 BOX 07/26/2017 1 NDC: 70069-072-01 1 in 1 CARTON 1 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207634 07/26/2017 Labeler - Somerset Therapeutics, LLC (079947873) Registrant - Somerset Therapeutics, LLC (079947873) Establishment Name Address ID/FEI Business Operations Wintac Limited 677236695 ANALYSIS(70069-072) , LABEL(70069-072) , MANUFACTURE(70069-072) , PACK(70069-072)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.