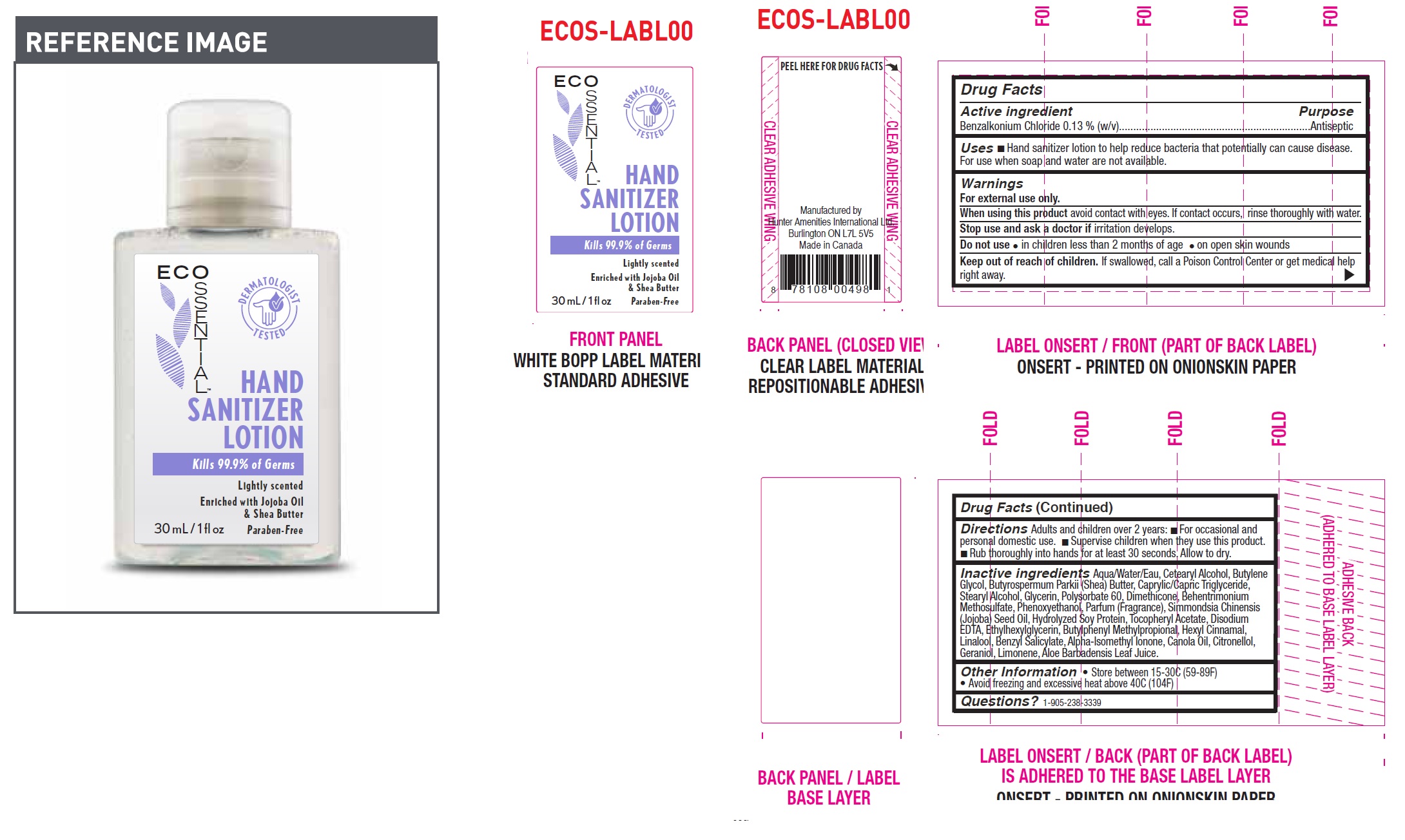
ECOSSENTIAL HAND SANITIZER LOTION
ECOSSENTIAL HAND SANITIZER by
Drug Labeling and Warnings
ECOSSENTIAL HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by Hunter Amenities International Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ECOSSENTIAL HAND SANITIZER- benzalkonium chloride lotion
Hunter Amenities International Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ECOSSENTIAL HAND SANITIZER LOTION
Uses
- Hand sanitizer lotion to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Directions
Adults and children over 2 years:
- For occasional and personal domestic use.
- Supervise children when they use this product.
- Rub thoroughly into hands for at least 30 seconds. Allow to dry.
Inactive ingredients
Aqua/Water/Eau, Cetearyl Alcohol, Butylene Glycol, Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Stearyl Alcohol, Glycerin, Polysorbate 60, Dimethicone, Behentrimonium Methosulfate, Phenoxyethanol, Parfum (Fragrance), Simmondsia Chinensis (Jojoba) Seed Oil, Hydrolyzed Soy Protein, Tocopheryl Acetate, Disodium EDTA, Ethylhexylglycerin, Butylphenyl Methylpropional, Hexyl Cinnamal, Linalool, Benzyl Salicylate, Alpha-Isomethyl Ionone, Canola Oil, Citronellol, Geraniol, Limonene, Aloe Barbadensis Leaf Juice.
| ECOSSENTIAL HAND SANITIZER
benzalkonium chloride lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hunter Amenities International Limited (244071015) |