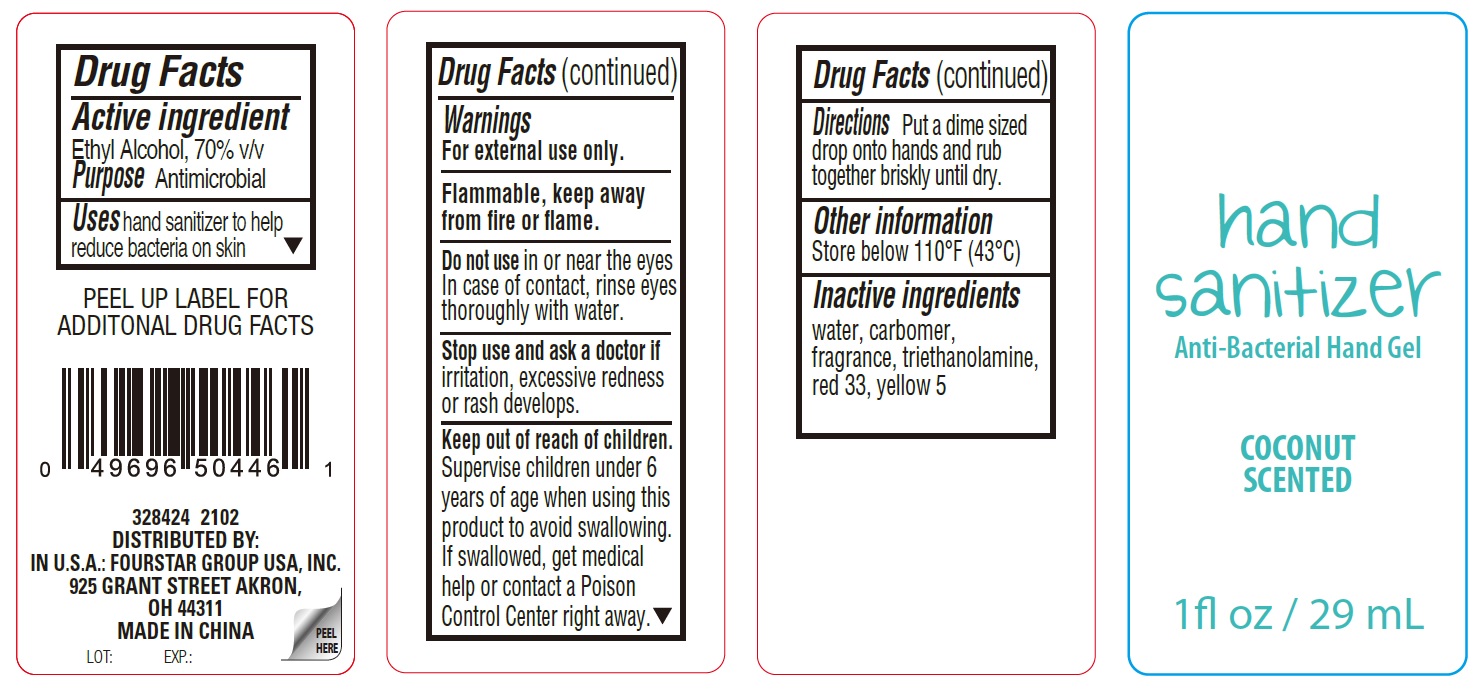
Coconut Scented Hand Sanitizer Gel
Coconut Scented Hand Sanitizer by
Drug Labeling and Warnings
Coconut Scented Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by FOURSTAR GROUP USA, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COCONUT SCENTED HAND SANITIZER- alcohol gel
FOURSTAR GROUP USA, INC.
----------
Coconut Scented Hand Sanitizer Gel
| COCONUT SCENTED HAND SANITIZER
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - FOURSTAR GROUP USA, INC. (140099503) |
Revised: 12/2023
Document Id: 0d6bf85c-3b4b-4b8d-e063-6294a90a728e
Set id: b5f86a7d-497a-2a3b-e053-2a95a90ae63c
Version: 3
Effective Time: 20231226